Biomedical applications of magnetic nanomaterials
QQ Academic Group: 1092348845
Detailed
(Northwestern University School of Chemistry and Materials Science, Faculty of Life and Medicine)
This article is selected from "Physics" Issue 6, 2020
Author: Chen Xiaoyong Liu Xiaoli Fan Haiming
Abstract Since scientists realized the controllable preparation of highly monodispersed nano-iron oxides at the beginning of this century, biomedical applications based on high-biological safety nano-iron oxides or doped nano-ferrite magnetic materials have seen explosive growth. At present, one of the frontier hot spots in the field of nanobiology and nanomedicine, especially its unique magnetic properties, enables magnetic nanomaterials to mediate external magnetic fields to produce nanoscale physical effects and act on micro-nano-scale biological targets. Through multidisciplinary research, it is expected to establish a new model of controllable, tissue-penetrable, safe and accurate disease diagnosis and treatment based on magnetic nanomaterials, which will improve the therapeutic effect of the disease and improve the prognosis. This article reviews some recent developments in the application of iron oxide particles in biomedicine, and mainly discusses the opportunities and developments of magnetic nanomaterials in new magnetic resonance imaging contrast agents, tumor magnetothermal therapy, and magnetic biological regulation.
Key words magnetic nanomaterials, magnetic resonance imaging, nanomagnetic biological regulation, magnetic induction heating
1 Introduction
In recent years, with the rapid development of nanoscience and technology and its cross-fusion with life sciences, medical engineering and other disciplines, many emerging disciplines and fields such as nanomedicine and nanobiology have emerged. Research in these intersecting fields is expected to provide new ideas and new methods for solving current technical challenges in the field of diagnosis and treatment of major diseases. Among many biomedical nanomaterials, magnetic nanobiomaterials have been widely used in clinical diagnosis and biomedical research due to their unique magnetic properties, such as tumor imaging diagnosis and magnetic hyperthermia, magnetic separation and instant testing, stem cells and regeneration MedicineBrain nerve stimulation and controlled drug delivery, etc. (Figure 1).
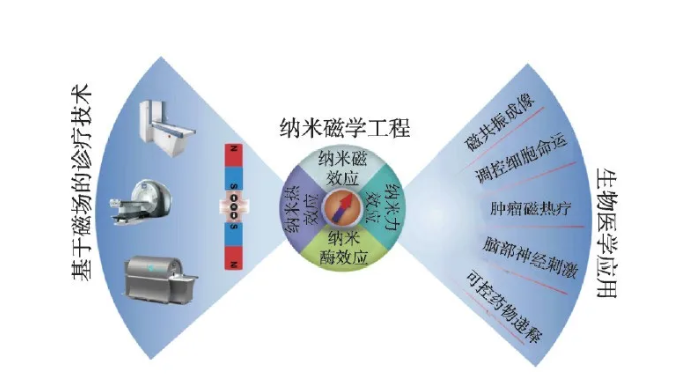
Figure 1 Biomedical applications of iron oxide nanoparticles
In addition to the use of biological effects related to the chemical composition and structure of nanomaterials, the main paradigm of biomedical applications of magnetic nanomaterials is to use the small size of nanomaterials to deliver them to specific biological targets, by applying safe and no tissue penetration The depth-limited external magnetic field stimulates magnetic nanomaterials to produce magnetic, heat, and force physical effects and act on biological targets [1-3], which in turn triggers a variety of biological effects to achieve diagnostic or therapeutic functions. It can be seen that magnetic nanomaterials are the medium for realizing the function of magnetic diagnosis and treatment, and its performance depends to a large extent on the coupling efficiency between the magnetic properties of the nanomaterials and the external magnetic field and the activation efficiency of the magnetic field-induced physical effects on the biological effects. . The ultimate goal of scientific research in this field is to realize a medical nanorobot that can be remotely magnetically manipulated in a living body, capable of early warning of diseases and following the instructions of doctors to perform related treatment functions. In order to better realize this long-term goal, it is necessary to deeply integrate the knowledge of multiple disciplines such as materials chemistry, nanomagnetism, biology, medicine and engineering, and gradually form new technologies such as "nanomagnetic medical engineering" in the process of continuous development. Interdisciplinary and direction.
Safety and effectiveness are the requirements for clinical applications of medical technology. Magnetic iron oxide particles are currently the only inorganic nano-medicine approved for clinical use, with excellent safety. The magnetic field-related medical technology often has the advantages of non-invasive, real-time control, and no radiation hazards. Therefore, from the perspective of clinical medical applications, interdisciplinary and innovative scientific research on the biological effects of iron oxide nanomaterials under the action of a magnetic field is expected to bring transformative medical technology to the biomedical field, improve current disease treatment effects, and meet the needs of Chinese people. Hope for health and a better life.
At the beginning of this century, scientists realized the controllable preparation of highly monodispersed iron oxide particles. After that, biomedical applications based on materials such as nano-iron oxide or doped ferrites have experienced explosive growth, including nanobiology and nanomedicine. One of the long-term research hotspots and frontiers in the field. In addition, the new biological effects of iron oxide particles that have been continuously discovered in research have further promoted the development of the forefront of biomedicine. For example, the discovery of the enzyme effect of iron oxide nanoparticles in 2007 triggered a new research field of "nanoenzymes" and promoted the development of technologies such as biological detection and medical treatment [4]. The magnetic induction heating effect mediated by iron oxide nanoparticles has also made breakthroughs in tissue freezing and resuscitation, body regulation of embryonic stem cells in the treatment of diabetes, and controlled release of drugs. In recent years, a large number of biomedical applications of iron oxide nanoparticles have been reviewed, but their frontier development in biomedicine mediated by magnetic fields remains to be summarized. Therefore, this article reviews the current status and progress of biomedical applications based on the magnetic, thermal, and force effects of nano-iron oxide generated under the magnetic field in recent years, especially in biomedical applications such as magnetic resonance imaging, tumor therapy, and cell function regulation. Opportunities and developments in the field, further discussing possible ways to deal with existing challenges.
2 Physical effects induced by magnetic fields
Magnetic properties are the key to the biomedical application of magnetic nanomaterials. Through material design, the magnetic properties of nanomaterials can be effectively controlled to improve application performance. Generally speaking, its magnetic performance parameters mainly include: saturation magnetization (Ms), magnetic anisotropy (K), remanence (Mr) and coercive force (Hc). Magnetization is a physical quantity that describes the magnetic strength of a macroscopic magnetic medium. For ferromagnetic ordered nano-iron oxide materials, the maximum magnetization that can be achieved with the continuous enhancement of the external magnetic field is the saturation magnetization. This parameter is related to the size, composition and morphology of the nano-material. For example, the saturation magnetization increases proportionally with the increase of the particle size within the threshold, and becomes constant after exceeding the threshold and close to the magnetization of the bulk material [5]. Magnetic anisotropy refers to the degree of difficulty of magnetization in different directions, including magnetocrystalline anisotropy, surface anisotropy and exchange anisotropy. The magnetocrystalline anisotropy is affected by the structure of the unit cell. For example, when Fe2+ in iron oxide nanoparticles is completely replaced by Co2+, the magnetic anisotropy is increased by 20 times compared with the original particles [6]. Surface anisotropy and exchange anisotropy are affected by the morphology of the particles. Generally, nanoparticles with a large specific surface area contribute more significantly to the surface anisotropy, and the core-shell structure also affects the exchange anisotropy [5, 7]. The coercivity can reflect the ability of the material to resist demagnetization, and is significantly affected by the change in particle size. When the grain size of nanomaterials is smaller than the size of a single domain, the coercive force increases with the increase of the grain size. It can be seen that ferromagnetic nanomaterials exhibit size-dependent magnetic properties. When the size is less than a certain critical value and its magnetocrystalline anisotropy (KV) is less than room temperature thermal energy (KT), its coercivity and remanence It tends to zero, showing superparamagnetic properties.
Magnetic nanomaterials can produce a variety of physical effects in response to external magnetic fields, and different applications have different requirements for the magnetic properties of the materials. Therefore, when designing high-performance magnetic nano-mediums, various factors need to be considered (Figure 2). Magnetic nanomaterials can generate a local induced magnetic field under the action of an external magnetic field. The local magnetic field can affect the hydrogen proton relaxation process excited by the radio frequency pulse, significantly shorten the relaxation time, and thereby enhance the magnetic resonance signal in the region. Nano-iron oxide is used as a typical T2 magnetic resonance enhancement contrast agent, and its relaxation efficiency is proportional to the square of the saturation magnetization. Under a gradient magnetic field or a rotating magnetic field, the magnetized nanomaterial can produce nanoscale mechanical effects, and its magnetic force is proportional to the product of the saturation magnetization and the external magnetic field gradient. In an external intermediate frequency alternating magnetic field (100-500 kHz), magnetic nanomaterials can convert the energy of the magnetic field into heat energy through hysteresis loss [8]. For superparamagnetic nanoparticles, the heat generation mechanism can be simplified as the sum of Brown and Neal relaxation losses. When the particle size is larger than the single domain size, the Brownian relaxation has a greater contribution to the heat generation; while the particles smaller than the single domain size are mainly contributed by the Neier relaxation, which is proportional to the magnetic anisotropy [9]. The magnetocaloric efficiency of magnetic nanomaterials can be evaluated by specific heat absorption rate (SAR) or intrinsic power loss (ILP). The heat generation efficiency is related to the frequency and intensity of the magnetic field and the saturation magnetization of the particles. The specific absorption rate does not consider the influence of magnetic field parameters, and the experimental results under different magnetic field parameters cannot be compared. The inherent power loss has nothing to do with the magnetic field parameters and can be used to measure the magneto-thermal conversion performance of materials under different external fields.
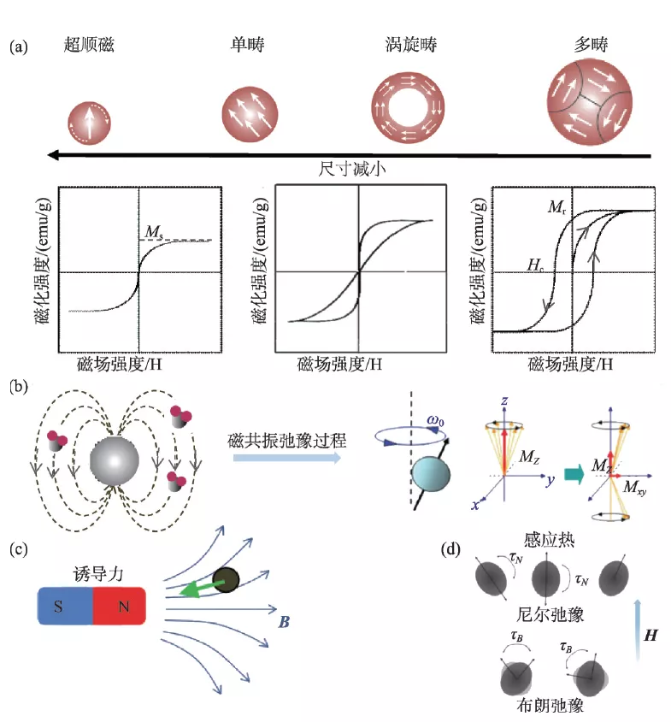
Figure 2 The size-dependent magnetic properties of nano-iron oxide and the basis of medical applications (a) Size-dependent hysteresis loop; (b) The effect of magnetic nanomaterials on hydrogen proton magnetic resonance relaxation; (c) Magnetic effect induced by magnetic field ; (D) Brown and Neal relaxation losses in the magnetic induction heating mechanism [10-12]
3 Magnetic resonance imaging contrast agent
At present, magnetic resonance imaging is widely used in clinical imaging diagnosis and disease monitoring. Contrast agent can enhance the signal of the lesion and improve its contrast with the surrounding normal tissues, thereby improving image sensitivity and early detection ability [13, 14]. Contrast agents generally enhance the contrast between normal tissues and diseased tissues by shortening the longitudinal relaxation time (T1 contrast agent) or transverse relaxation time (T2 contrast agent) of hydrogen protons. T1 contrast agent can make the target area brighter and is a positive contrast agent. T2 contrast agent makes the imaging of the diseased tissue darker and belongs to a negative contrast agent. The relaxation enhancement efficiency of T1 and T2 contrast agents is measured by the relaxation rates r1 and r2, respectively. Generally speaking, to obtain a larger r2 value, the nanoparticles are required to have a higher magnetization intensity. For example: Fe3O4 nanorings have a large magnetic resonance r2* value due to their high magnetization and high magnetic field inhomogeneity, which is 4 times stronger than traditional superparamagnetic iron oxide [15]. However, T2 imaging is easily confused with low-intensity areas such as bleeding, calcification, and metal deposition, leading to missed diagnosis and misdiagnosis of lesions during clinical detection. Therefore, the development of a new T1 contrast agent based on nano-iron oxide is expected to improve the diagnostic performance of magnetic resonance imaging, and it is also one of the current research hotspots.
3.1 Iron oxide T1 contrast agent
Paramagnetic ions are the key to the enhancement of MRI T1 signal. Both free Fe2+ and Fe3+ paramagnetic ions can lead to the enhancement of T1 signal. For nano-iron oxide, as its size decreases sharply, the proportion of paramagnetic iron ions on the surface increases significantly. The small size also suppresses the magnetization and T2 relaxation efficiency of the particle core; when the size is reduced to 5 nm Below, the core-shell structure nano iron oxide composed of a high proportion of surface paramagnetic amorphous layer and internal very small ferromagnetic crystal cores shows quasi-paramagnetic properties as a whole, with high r1 relaxation rate and low r2/ The r1 ratio can be used as a new and low-toxic T1 contrast agent. In 2002, Taupitz et al. evaluated citric acid-modified 4 nm iron oxide VSOP-C91 as a T1 contrast agent for magnetic resonance angiography, and the results showed that ultra-small iron oxide can exhibit better vascular T1 signal enhancement [16]. Even so, the preparation of highly monodisperse ultra-small iron oxide was still a challenge at the time. In 2011, the Hyeon research group of Seoul University in South Korea used iron oleate as a precursor and oleyl alcohol as a reducing agent to prepare monodisperse ultra-small iron oxide nanoparticles (1.5 nm, 2.2 nm, 3 nm) in a benzyl ether solution on a large scale. , And used 3 nm as a model system to evaluate its in vivo vascular imaging effect [17]. Subsequently, the research group also used large animal models such as beagles and rhesus monkeys to evaluate the safety of the contrast agent and the diagnostic effect of cerebral ischemia. These results indicate that ultra-small iron oxide nanoparticles are expected to become the next generation of efficient and safe magnetic resonance contrast agents [18]. However, the T1 relaxation rate of the ultra-small nano-iron oxide contrast agent is only 4.6 s-1·mM-1, which has no advantage over the relaxation efficiency of clinical gadolinium-based contrast agents. In addition, because the small size can be controlled and prepared, the development of nano-contrast agent relaxation enhancement theory is lagging behind, and the magnetic structure of ultra-small ferrite and the T1 signal enhancement mechanism are still unclear, so it is difficult to optimize its performance.
In 2017, our research group used the thermal decomposition properties of metal carboxylic acid complexes to propose a universal dynamic simultaneous thermal decomposition method and successfully prepared a series of highly monodisperse ultra-small (less than 4 nm) ferrite nanoparticles . Secondly, by analyzing the relationship between composition, crystal structure and magnetic properties, the "quasi-paramagnetic" spin-ordered magnetic structure formed by its ferromagnetic crystal core and paramagnetic amorphous shell layer is clarified. Furthermore, a systematic study of the effects of different ferromagnetic crystal nuclei and paramagnetic shells in the quasi-paramagnetic structure on the T1 relaxation enhancement reveals that the T1 relaxation enhancement is not dominated by a single internal sphere relaxation mechanism that is generally believed to be dominated. The inner sphere relaxation dominated by the shell and the outer sphere relaxation dominated by the nucleus are synergistically enhanced. The quasi-paramagnetic nano-manganese ferrite (UMFNP) optimized according to this theory has a T1 relaxation rate as high as 8.43 s-1·mM-1, which is twice as high as that of clinical gadolinium-based contrast agents. In vivo imaging experiments have proved quasi-paramagnetic High-definition T1 vascular imaging performance of manganese ferrite (Figure 3) [19]. In 2019, we further systematically evaluated the relationship between manganese content in ultra-small manganese ferrite nanoparticles and liver T1 contrast enhancement performance in vivo, and revealed the liver-specific magnetic resonance imaging mechanism mediated by its transmembrane metal transport protein SLC39A14. Research shows that with the increase of manganese content in ultra-small manganese ferrite, r1 first increases (MnxFe3-xO4, x = 0.75), and then gradually decreases (x = 1.57). However, with the increase of manganese content, the degree of enrichment of manganese ferrite particles in the liver also increases. Excessive manganese content will also reduce the cell survival rate and affect the liver‘s excretion and secretion functions to a certain extent[ 20].
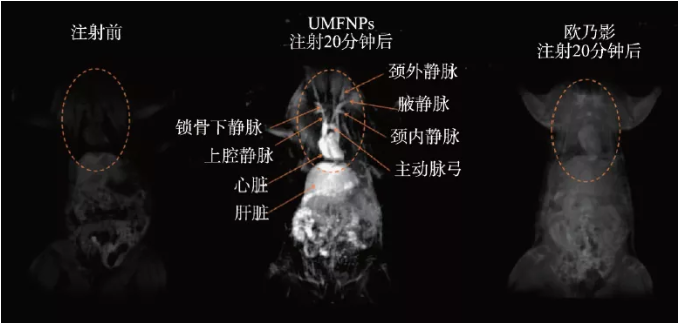
Figure 3 In vivo quasi-paramagnetic manganese ferrite (UMFNP) enhanced magnetic resonance image [19]
3.2 Iron oxide T1/T2 dual-modal contrast agent
Traditional iron oxide nanoparticle magnetic resonance contrast agents are usually used as single-modal contrast agents, while single T1 or T2 mode imaging is prone to false positive signals due to endogenous factors such as fat, calcification, hemorrhage, blood clots and air. It is difficult to accurately present the target area of the diseased tissue. The development of T1/T2 dual-modal probes and the use of dual-modal magnetic resonance imaging can greatly enhance the accuracy and sensitivity of its imaging diagnosis. At present, there are two main methods for constructing T1/T2 dual-mode iron oxide nanoprobes based on iron oxide: one is to dope paramagnetic elements into nano-iron oxide particles. For example, doping europium in iron oxide nanoparticles can Synthetic T1/T2 contrast agent, its T1 and T2 relaxation efficiencies are significantly better than Fe3O4 or Eu2O3 nanoparticles [21]; the second is to achieve the two modes by optimizing the size and surface of iron oxide nanoparticles, or forming a core-shell structure Compatible with imaging. The probes constructed by these methods can obtain dual-modal contrast-enhanced imaging by simply using T1 or T2 sequences, achieving self-confirmation cross-validation of diagnostic accuracy [17].
Different from the above two methods, quasi-paramagnetic ultra-small nano-iron oxide provides a new imaging mode of T1/T2 enhanced dynamic transformation for magnetic resonance imaging. Due to the unique quasi-paramagnetic properties of ultra-small nano-iron oxide, the well-dispersed ultra-small nano-iron oxide exhibits enhanced T1 contrast. However, the agglomerated ultra-small nanometer iron oxide exhibits superparamagnetism and enhanced T2 contrast due to the magnetic dipole interaction between the particles. Based on this principle, using endogenous stimuli such as weakly acidic pH, enzymes, redox, etc. of tumor tissues to promote the change of quasi-paramagnetic nanoprobes from agglomeration to dispersion or the opposite process, the enhancement of T2 to T1 or T1 to T2 can be achieved Dynamically variable magnetic resonance imaging. Ling et al. synthesized a pH-sensitive magnetic iron oxide agglomerate nanoprobe, which induced the change from aggregate to dispersed nanoparticle through pH, and realized the transition from T2 to T1 contrast effect [22]. Wang et al. used the self-assembly behavior of sub-5 nm ultra-small iron oxide nanoparticles in the acidic microenvironment of tumors to realize the conversion of T1-weighted contrast to T2-weighted contrast [23]. Jia et al. synthesized ultra-small Fe3O4 nanoprobes modified with integrin (RGDyK) [24, 25]. The suitable hydrodynamic size allows it to gradually accumulate in the tumor stroma, which makes the magnetic resonance signal from T1-weighted comparison The effect (diffusible nanoparticles) is transformed into a T2-weighted contrast effect (aggregated nanoparticles), and shows a dependence on time.
3.3 Multi-mode imaging probe based on iron oxide
Magnetic resonance imaging has limitations in its application such as difficulty in accurate quantification and relatively low sensitivity. The design of multi-mode nanoprobes can achieve integration and complementary advantages with other imaging methods, thereby improving the sensitivity, accuracy and quantitative ability of diagnosis . For example, Li et al. prepared Fe3O4/Au nanoparticles through a hydrothermal method in one step and used them for magnetic resonance-electron computed tomography (MRI-CT) dual-modality imaging [26]. The nanoparticles have high T2 relaxation efficiency, good X-ray attenuation characteristics and a high Fe3O4/Au molar ratio, which significantly improves the accuracy of imaging. Choi et al. modified the tyrosine residues of serum albumin on manganese ferrite nanoparticles for magnetic resonance-positron emission tomography (MRI-PET) [27]. The resulting dual-contrast agent obtains highly sensitive signals in both MRI and PET images, and further fusion analysis of the two images can accurately detect different types of tiny sentinel lymph nodes at the millimeter level. Sun et al. used water/oil/water (W/O/W) double emulsion method to encapsulate iron oxide nanoparticles in polylactic acid-glycolic acid copolymer (PLGA) microcapsules to achieve choline anaplastic squamous rabbit liver cancer cells (VX2) the simultaneous enhancement of in vivo magnetic resonance imaging and ultrasound imaging signals [28]. Du et al. successfully developed a kind of magnetic ferrite nanoparticles (MFNPs), and for the first time realized the high-efficiency magnetic hyperthermia guided by the in vivo dual-mode magnetic resonance-magnetic particle (MRI-MPI) image of nanoparticles for malignant tumors. The integration of magnetic diagnosis and treatment provides a new strategy [29]. At present, through the design of multifunctional nanoprobes based on iron oxide, different imaging technologies such as single photon emission computed tomography (SPECT), fluorescence imaging (FLI), photoacoustic imaging (PAI), etc. are combined with magnetic resonance imaging. Multi-mode precision imaging has attracted more and more attention from researchers [30].
4 Biomedical applications based on magnetism
Magnetic nanoparticles can produce mechanical effects in a gradient magnetic field or a rotating magnetic field. This "nanomagnetic force" can be used to regulate cell function and fate, such as destroying the cell membrane system or cytoskeleton, leading to cell apoptosis or necrosis. Shen et al. reported that magnetic nanoparticles and epidermal growth factor (EGF) can self-assemble to form a magnetic knife (MNPS-EDF) under the action of a low-frequency field, resulting in the rupture of lysosomal membranes and cell membranes, resulting in more than 90% cell death (Figure 4) )[31]. Master et al. found that the application of an external field can make the nanoparticles (7-8 nm) that are endocytosed into the lysosome rotate, causing damage to the microfilaments and destroying the cell structure [32]. Nanomagnetic force can also be used to promote the differentiation of stem cells and the formation of functional tissues. For example, Kang et al. controlled iron oxide nanoparticles and arginyl-glycyl-aspartic acid (RGD) complexes (SPIO-RGD) by applying external fields of different frequencies. ) Oscillation rate, which realizes the remote manipulation of macrophage adhesion and polarization phenotype in vivo/external [33]. Hu et al. proved that magnetomechanical stimulation has a significant effect on the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells (hMSCs) [34].
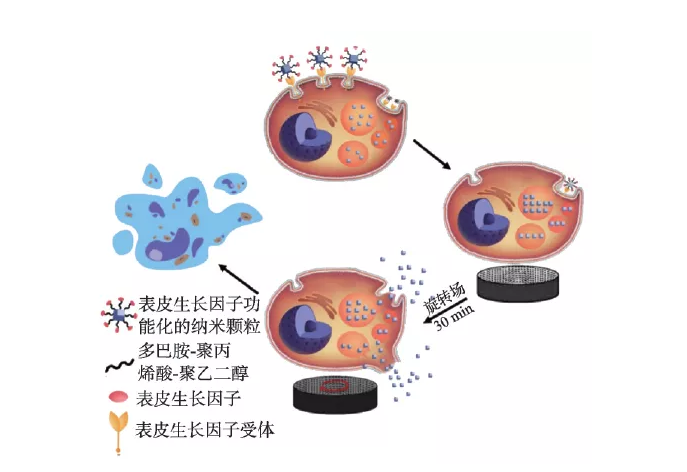
Figure 4 The destruction mechanism of lysosomal membrane and cell membrane under low-frequency magnetic field [31]
The cleverly designed micro-nano magnet structure can also realize the development of magnetically driven nanorobots with high biocompatibility. Hu et al. developed a magnetic soft robot whose main body is composed of silicone shell and neodymium iron boron material. This kind of soft robot can change its shape through time-varying external magnetic field control, and realize swimming and climbing inside and on the liquid surface, or rolling, walking, and crossing obstacles on solid surfaces [35]. Cui et al. magnetically programmed a single-domain nano-cobalt magnet array on a flexible silicon nitride (Si3N4) sheet substrate to encode multiple deformation instructions into a micromachine (Figure 5(a), (b)). When the programmed nanomagnet is exposed to an external magnetic field, the magnetic force drives the component to move. By constructing a micron-scale "artificial bird", flying actions such as flapping, hovering, turning and gliding under the drive of a magnetic field are realized (Figure 5(c), (d)) [36]. Magnetically steerable nanorobots can theoretically achieve targeted drug delivery, biopsy and cardiac stent placement in tiny blood vessels, and its further development is expected to bring a disruptive breakthrough in the field of minimally invasive medicine.
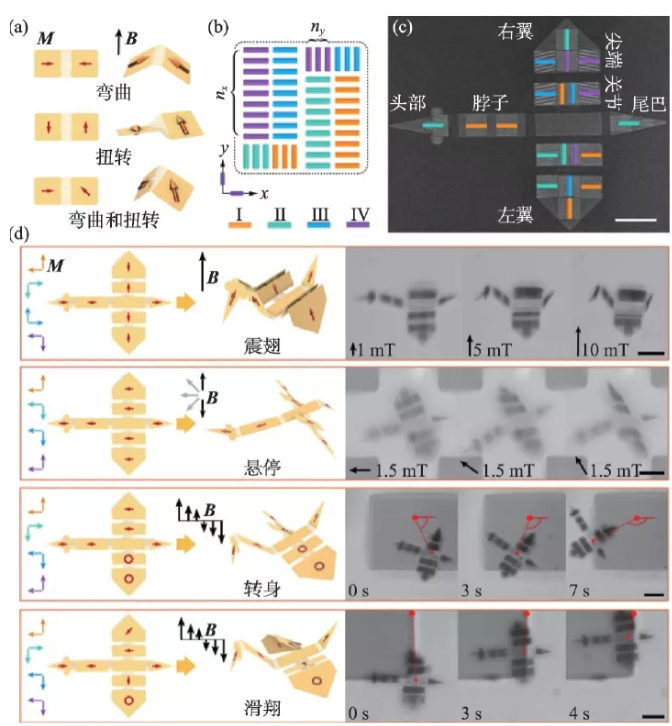
Figure 5 Origami-like miniature artificial bird with multiple deformation modes[36] (a) Schematic diagram of the folding behavior of a double-panel device when driven by a magnetic field; (b) a schematic diagram of the panel design of nano-magnetic materials; (c) a miniature bird SEM image; (d) Miniature birds mimic 4 flight modes
5 Biomedical applications based on magnetic induction heating
5.1 Tumor magnetic hyperthermia
Magnetic hyperthermia (MTT) is a new type of anti-tumor physical therapy. It uses the heat-generating effect of magnetic nanoparticles under the action of an alternating magnetic field and the characteristics of tumor cells that are less thermally resistant than normal cells. Magnetic nanoparticles are injected into the tumor site, and then an alternating magnetic field is applied to selectively kill tumor cells. Since the concept of tumor magnetic hyperthermia was proposed in 1957, it has been developed for more than 60 years, especially in the past 10 years, thanks to the rapid development of nanomaterials technology, the therapeutic effect has been significantly improved. At present, the treatment has been certified by the European Union for clinical treatment , And completed the clinical registration of prostate cancer treatment in the United States, which is the current research focus of nanomedicine and translational medicine. At present, biomedical magnetic nanoparticles are usually superparamagnetic iron oxide nanoparticles with a size of less than 20 nm. However, their small size and weak magnetic properties result in low magneto-thermal conversion efficiency (SAR about 250 W/g), which seriously restricts tumors. The effect of magnetic heat treatment. Adjusting and controlling the particle size [37], composition [38], morphology [5, 39] and surface modification [40] of nano-iron oxide are currently the main means to improve the efficiency of magnetocaloric conversion. For example, Lee et al. adjusted the K value by forming soft magnetic and hard magnetic core-shell structures, which greatly increased the specific power loss (SLP) value of nanoparticles. The specific power loss of CoFe2O4@MnFe2O4 is as high as 2280 W/g, which is much higher than the hard magnetic material CoFe2O4 (443 W/g) of 9 nm and the soft magnetic material of 15 nm (411 W/g) [9]. Noh et al. found that 18 nm cubic nanoparticles Zn0.4Fe2.6O4 have a smaller surface spin disorder layer (4%) than spherical nanoparticles (8%), resulting in a cube with higher magnetocaloric conversion efficiency than spherical [5] . In recent years, our research group has developed a new type of iron oxide nanoring with vortex magnetic domain structure. Due to its small size boundary effect and special structure, its magnetic moment is distributed in a vortex in the clockwise or counterclockwise direction in the plane. , Forming a unique multi-domain structure with closed magnetization distribution. It has both the sol dispersibility of superparamagnetic iron oxide nanoparticles and the vortex-onion magnetization reversal induced by the external field, which leads to large hysteresis loss and excellent magnetic properties close to bulk materials. Under the action of an alternating magnetic field, its magneto-thermal conversion efficiency is an order of magnitude higher than that of superparamagnetic nanoparticles. Taking tumor-bearing mice as a model, the magneto-thermal treatment time is shortened from 60 minutes in clinic to 10 minutes, and the dosage is from 5-10 mg Fe/kg tumor tissue is reduced to 0.5 mg Fe/kg tumor tissue, which proves that materials with high magneto-heat conversion efficiency can significantly improve tumor magneto-thermal therapy technology, reduce dosage and toxic side effects [41].
Previous studies believed that the killing of tumor cells by magnetic hyperthermia mainly depends on the thermal effect, that is, the macroscopic temperature of the tumor site must be above 43 ℃ to kill the tumor cells. Therefore, the macroscopic thermal effect has become a predictor of the tumor suppression efficiency and the curative effect of magnetic hyperthermia. Key indicators. Recent studies have shown that the microscopic thermal effect of nanoparticles in cells may be the main reason for their efficacy. The micro- and nano-scale thermal effects mediated by nano-iron oxide can not only regulate protease activity, but also significantly enhance reactive oxygen species (ROS) in the tumor hypoxic microenvironment, thereby achieving high-efficiency inhibition of solid tumors and their metastases. An in-depth understanding of the biological effects of reactive oxygen species generated in the magnetocaloric process mediated by nano-iron oxide is of great significance to the realization of precise magnetic therapy. For example, it has been reported that the increase of reactive oxygen species in the tumor microenvironment can promote the immune response to attack tumor cells, but this effect is completely ignored in traditional magnetic hyperthermia. We recently combined the magnetic induction heating effect of nano-iron oxide with its immune effect related to the induction of reactive oxygen species, and proposed a new type of magneto-thermodynamic therapy (MTD), which breaks through the limitations of traditional magnetic hyperthermia and effectively inhibits tumor growth. [42]. First, vortex magnetic iron oxides (FVIOs) coupled through nanographene oxide sheets (GO) to form FVIOs-GO nanocomposites. The nano material can enhance the magnetothermal conversion efficiency and promote the production of active oxygen under the stimulation of the alternating magnetic field. The results of in vitro and in vivo experiments showed that under the conditions of hypoxic tumor microenvironment and physiologically tolerable temperature (~40 ℃), the active oxygen enhanced by magnetocaloric triggers a significant immune response (Figure 6); Magneto-thermodynamic therapy can expose calreticulin on the surface of 83% of 4T1 breast cancer cells, directly promote the polarization of macrophages to the pro-inflammatory M1 phenotype, and increase tumor infiltrating T lymphocytes. Furthermore, the results of the intravenous anti-tumor experiment of the 4T1 subcutaneous breast tumor model showed that under the dual effects of thermal effects and reactive oxygen-related immune effects, the therapy used a low dose (3 mg/kg) and a shorter exposure time to alternating magnetic fields. (2 times, a single 10 minutes) can effectively inhibit tumor growth, while traditional magnetic hyperthermia requires 4-8 alternating magnetic field treatments at a dose of 6-18 mg/kg to achieve similar effects. The proposal of magneto-thermal kinetic therapy not only promotes the development of traditional tumor magneto-hyperthermia, and overcomes its shortcomings of relying only on thermal effects, but also deepens the understanding of the mechanism of nano-iron oxide-mediated tumor magneto-hyperthermia; combined with active oxygen The mediated immune effect not only significantly improves the anti-tumor efficacy, but also provides new ideas for precise and efficient nanomagnetic therapy in the future.
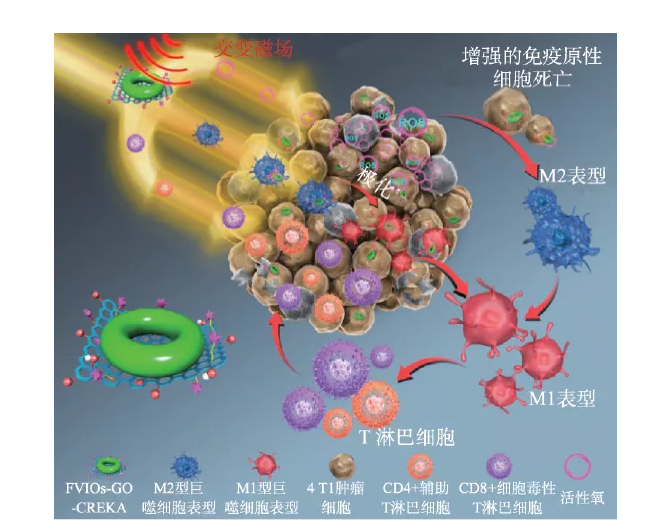
Figure 6 FVIO-GO-mediated magneto-thermodynamic therapy mechanism [42]
5.2 Magnetothermal treatment
Magnetic hyperthermia can not only kill tumor cells by inducing heat generation, but can also be used as an adjuvant therapy for clinical tumor radiotherapy and chemotherapy, as well as synergistic tumor photothermal/photodynamic therapy, immunotherapy, etc. [43,44]. Magnetic particles loaded with drug molecules can not only improve the toxic and side effects of chemotherapeutic drugs, but also achieve controlled magnetic and thermal release of drugs under an alternating magnetic field and improve the efficacy of chemotherapy. For example, Ren et al. developed a nanomedicine (Fe3O4-MNPs-DNR) that combines the chemotherapy drug daunorubicin (DNR), P-glycoprotein inhibitor 5-bromotetrandrine (5-BrTet) and magnetic nanoparticles. -5-BrTet), effectively inhibited the growth of xenogeneic tumors in nude mice with leukemia and resulted in a significant down-regulation of the expression of P-glycoprotein [45]. Magnetic induction heating can also play an important role in the sensitization process of radiotherapy. The physical heating effect can interfere with the repair of damaged tumor DNA and enhance the damage of radiotherapy to tumor cells and blood vessels. Espinosa et al. designed a gadolinium-doped iron oxide nanoparticle with high heat conversion efficiency and found that hyperthermia can increase radiotherapy by reducing the proportion of hypoxic cells (with radiotherapy resistance) and inducing tumor-specific local vascular rupture and necrosis. Effective [46]. In addition, the combination of magneto-hyperthermia and photo-hyperthermia can provide a cumulative thermal effect and achieve a synergistic anti-tumor effect of 1 + 1> 2. For example, Ma et al. prepared Fe3O4-Pd nanoparticles and irradiated them in an intermediate frequency magnetic field and near-infrared light. Under this condition, the synergistic enhancement effect of "magneto-heat + light-heat" can be realized at the same time, and the production of active oxygen is enhanced. The experimental results of the 4T1 in situ breast tumor model showed that the enhanced thermal conversion efficiency and ROS based on the nanoparticles can effectively inhibit tumor growth [47]. Magnetic hyperthermia can also be effectively synergized with immune checkpoint therapy. By activating the anti-tumor immune response, it can enhance the infiltration of CD8+ cytotoxic T lymphocytes of distant tumors, so that 4T1 triple negative breast cancer cells can block PD-L1 checkpoint treatment Sensitization increases the population benefiting from immunotherapy; the combination therapy can also change the tumor immunosuppressive microenvironment at the same time, such as significantly down-regulating myeloid-derived suppressor cells (MDSCs), inhibiting tumor metastasis and improving prognosis [43]. Clinical practice has shown that comprehensive treatment is the most effective for tumors. Magnetic hyperthermia is combined with a variety of tumor treatment methods and enhances its curative effect. It has good clinical transformation value. Further in-depth study of the synergistic mechanism between different therapies can lay the foundation for the complete cure of clinical tumors in the future.
5.3 Other biomedical applications
Nano-material-mediated magnetic induction heating can not only be used for tumor treatment, but also be applied to heat-stimulated drug release, brain nerve regulation, and biological tissue freezing and resuscitation. For example, Hu et al. reported a core/shell structured nanocarrier. The drug-loaded silica core is wrapped by a single crystal iron oxide shell. The iron oxide shell can prevent the diffusion and leakage of the drug. When the nanocarrier is subjected to a high-frequency alternating magnetic field , Nano-scale cracks appear in the edge area of the thin iron oxide shell, and the controlled release of drug molecules driven by magneto-thermal is realized [48]. Chen et al. used the magnetocaloric effect of iron oxide nanoparticles in an alternating magnetic field to activate the thermosensitive capsaicin receptor TRPV1 of neurons and induce calcium ion influx in neurons to achieve projections to the ventral tegmental area of mice. Long-distance, wireless magnetocaloric controllable regulation of the excitability of neuronal subgroups in the brain [49]. In addition, the research alsoIt is found that the magnetocaloric effect can meet the requirements of rapid and uniform heating when tissues or organs are thawed during freezing and resuscitation of biological tissues. For example, Manuchehrabadi et al. dispersed silica-coated iron oxide nanoparticles in biological tissue freezing liquid and applied an alternating magnetic field to achieve rapid heating of the freezing liquid (rate of about 100-200 ℃/min) and uniform biological tissue. Safe heating and thawing recovery [50].
6 Summary and Outlook
Magnetic iron oxide was recorded as a medicine in the "Shen Nong‘s Materia Medica" as early as the Eastern Han Dynasty, and magnetic medicine, which uses magnetic fields for diagnosis and treatment, has a long history in China. Therefore, the new medical technology developed based on the physical effects of nano-iron oxide-mediated magnetic fields not only has the historical heritage of traditional medicine, but also is a new starting point for the development of modern multi-disciplinary cross-integration. Due to space and time constraints, the article only briefly discusses some recent developments in this field. Although it fails to give a complete picture, it should be possible to outline its basic development. Existing studies have clearly shown that biomedical applications based on magnetic nanomaterials have great potential, but there are still many problems that need to be resolved if clinical translation is to be carried out: (1) Starting from basic research, design safety through nanomagnetic engineering , High-performance nano-magnetic materials, to further improve its application performance; (2) For basic research and clinical transformation, develop simple and controllable magnetic field generation equipment and control systems; (3) Clarify the physical effects and biological effects of nano-iron oxide-mediated magnetic fields The mechanism of the target, etc. Finally, strengthen the training of multidisciplinary talents, establish an interdisciplinary research funding system and evaluation system, so as to effectively promote the development of related fields and promote the connection of basic research and applied research. This is also the only way for future scientific development.
Source of information: Journal of the Chinese Physical Society
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
- Previous: Summary of some resear
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier