ACS Nano: MXene must read! Catalysis & energy storage shine
QQ Academic Group: 1092348845
Detailed
【Research Background】
Electricity, light, and photoelectric catalysis play a vital role in realizing a sustainable energy economy. It can promote a large number of redox reactions in energy storage and conversion systems, allowing the production of chemical raw materials and cleanliness from abundant resources such as water, carbon dioxide and nitrogen. Fuel becomes possible. However, a major obstacle to large-scale applications is the lack of low-cost, durable and efficient catalysts. Recently, the family of two-dimensional transition metal carbides, nitrides and carbonitrides (MXenes) has become a major player in large-scale catalytic energy storage and conversion due to its unique hydrophilicity, high conductivity and easy liquid-phase processing and production characteristics. A highly potential choice. In order to make full use of these satisfactory properties, MXenes are combined with other materials to form MXene hybrids, which can significantly improve its catalytic performance.
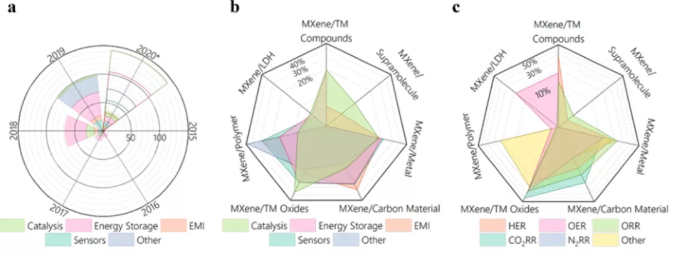
Figure 1 Application statistics of MXenes and its hybrid materials.
[Article Introduction]
Recently, Indiana University-Purdue University-Indianapolis, Babak Anasori and Zhi Wei She, Singapore’s Science and Technology Research Agency, collaborated on “Rational Design of Two-Dimensional Transition Metal Carbide/Nitride (MXene) Hybrids and Nanocomposites for Catalytic Energy Storage and "Conversion", published a review article on ACS Nano. The author outlined the latest design strategies of MXene hybrid materials for industrial-related electrocatalysis, photocatalysis and photocatalysis applications. At the same time, the current lack of understanding of MXene-based hybrid materials is emphasized to guide the future application of MXene-based hybrid materials in catalytic energy storage and conversion.
[Article Interpretation]
1. Design strategy of MXene hybrid/composite materials
The layered structure and surface diversified Tx functional groups of MXenes provide highly adjustable properties and strong compatibility with other materials, thereby realizing MXene hybrids with high catalytic performance. The wide variety of other materials compatible with MXene (transition metal compounds, supramolecular structures, carbon-based materials, and metals), coupled with the different synthesis methods to form these hybrid materials (Figure 2), shows that these hybrid materials are satisfying The potential for energy-related applications and various needs in other fields is unlimited. The synthesis strategy of MXene hybrid materials is explained below from the perspective of synthesis method.
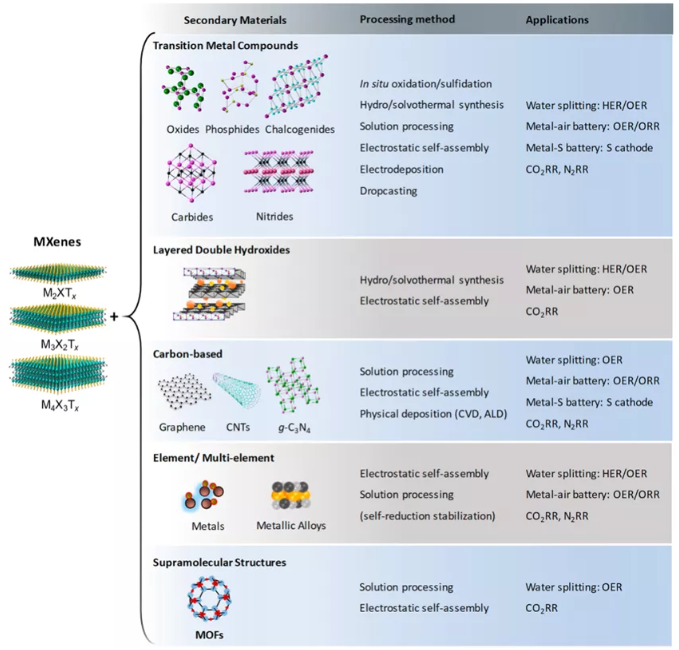
Figure 2 Three different types of MXene (M2XTx, M3XTx and M4XTx) are combined with other materials to form MXene hybrid materials.
1.1 In-situ conversion reaction of MXene surface to produce hybrid materials
The layered structure of MXenes exposes the transition metal atomic layer on their outer base (left image in Figure 2). These transition metal layers are highly oxygen-philic and are therefore prone to surface reactions such as oxidation or sulfidation (Figure 3). During these processes, the MXene surface is partially or fully transformed to form the second phase material. Therefore, this process can be called the in-situ transformation of MXene surface. The advantages of surface transformation are obvious: the second material is tightly combined with MXenes to form a mixed interface. Since the MXene surface directly participates in the conversion, the growth of the second material does not require an additional transition metal source. Therefore, this process is self-limiting, reducing the transitional nucleation and growth of the second phase material, otherwise the catalytic effect of the material will be limited.
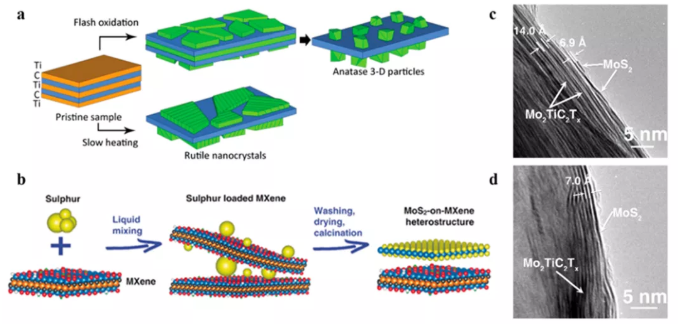
Figure 3 In-situ transformation of MXene surface to form MXene hybrid material. (a) The oxidation mechanism of Ti3C2 to TiO2 under different oxidation conditions. (b) Heat treatment of Mo2TiC2 vulcanized at different temperatures, and cross-sectional transmission electron microscope images: (C) 500°C and (d) 700°C.
1.2 In-situ reaction of MXene surface to produce hybrid materials
Compared with the in-situ conversion of MXene surface, this method allows a wider range of material components to be composited without relying on the surface transition metal properties of MXene. Here, MXenes can be regarded as the substrate for the growth of the second phase material, rather than the reactant. The main advantage of in-situ reaction formation is to maintain the integrity of MXenes during the synthesis process, because almost any precursor (cation, anion or metal) can be used for the nucleation and growth of secondary materials. In this way, a mixture with a high second phase material/MXene ratio can be formed. Therefore, if the second phase material is catalytically active, this strategy is most useful because it can grow in large numbers on MXene with a large surface area. Typical synthetic routes that can be used for this strategy include water/solvothermal synthesis, liquid phase treatment, and deposition (physical, electrical or light induced). In addition to physical deposition, most of these synthetic routes rely on van der Waals or ionic/electrostatic interactions between the negatively charged MXene surface (from the Tx functional group) and the positively charged second phase material precursor. After the initial adsorption, the second phase material is formed in situ on the surface of MXene by chemical treatment or heat treatment.
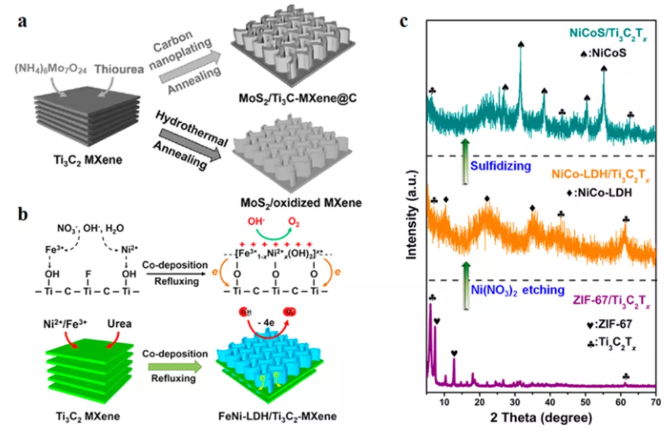
Figure 4 Forms the second phase material in situ on the surface of MXene. (A) During the hydrothermal treatment process, carbon nano-sprayed to resist oxidation; (b) In-situ electrostatic self-assembly of FeNi LDH/Ti3C2Tx hybrid; (c) XRD spectrum.
1.3 Non-reactive assembly of the second phase material on MXene
The second phase material with positive surface charge can also be easily combined with MXene through a physical process to form an MXene hybrid material. This method also relies on the same electrostatic/van der Waals self-assembly principle as the previous method. However, in this non-reactive method, the second phase material is in its final form before being compounded with MXenes. Therefore, MXenes usually do not undergo high temperature treatment steps, such as water/solvent heat treatment. This method is most suitable for the second phase material that can be processed in liquid phase and is stable at room temperature.
1.4 Chapter summary
1) The synthesis method selected for the formation of MXene hybrid material is highly dependent on its expected catalytic application field;
2) The MXene hybrid material should be carefully designed to optimize the interface chemical coupling between MXene and the second phase material to improve the catalytic activity;
3) In various synthesis methods, the Tx functional group on the surface of MXene plays an important role in the morphology, phase and growth characteristics of the second phase material.
2. MXene hybrid material for water splitting reaction
The unique physical and electronic properties of MXenes make it an important candidate for catalytic water splitting. High hydrophilicity and solution processability make MXenes stable and durable in acidic, alkaline and seawater. The high electronic conductivity allows MXenes to promote effective electronic charge transfer at the electrode-electrolyte interface as a large-area conductive 2D substrate when combined with other highly active but less conductive promoters. The combination of these properties overcomes the limitations of conventional carbon-based supports, such as graphene. The hydrophobicity of graphene limits its application in hydroelectric catalysis. Hybridization also generates additional catalytically active elements at the interface and adjusts the electronic structure to enhance intrinsic catalytic activity.
2.1 MXene hybrid/composite materials for electrocatalytic HER
After theoretically predicting MXenes and proving that it has HER activity, MXenes has attracted great attention. Unlike other 2D materials (such as 2H-MoS2), the 2D base surface of MXenes has higher HER activity and can use catalysts more effectively. Sehet et al. used Mo2CTx and Ti2CTx as examples to test the activity of HER, where the -O group was identified as the active site on the MXenes base. Due to the moderate electrocatalytic HER activity of pure MXenes, the initial efforts aimed to physically increase the density of electrochemically active surface area through nanostructure design or by adjusting the electronic structure of MXene, thereby obtaining more thermally neutral *H adsorption free energy (△GH→0). Effective binding energy adjustment can be achieved through Tx functional group control and doping with other transition metals or non-metals (such as nitrogen, sulfur, and phosphorus).
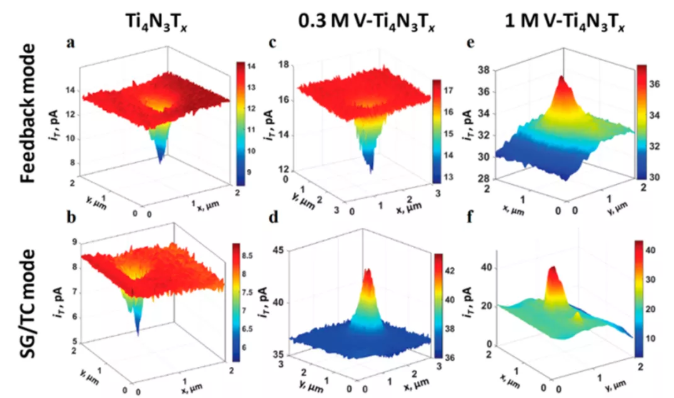
Figure 5 (ab) Scanning electrochemical microscope feedback mode and substrate generation/tip collection (SG/TC) drawing of pure Ti4N3Tx and Ti4N3Tx of V alloy, using (c, d) 0.3 M or (e, f) 1.0 M water system VOSO4 dopant alloying.
Coupling MXenes with other HER active promoters is a common method to enhance HER activity, such as transition metal sulfides, phosphides, carbides and quantum dots (metal alloy and black phosphorus, BP). Here, MXenes act as a large-area conductive and mechanical carrier to promote charge transfer, act as a HER promoter, and adjust the electronic structure of the hybrid material to optimize H+ adsorption and HER activity. Since the activity of HER is significantly higher than the sum of its individual components, the tightly bound MXene hybrid material is enhanced by synergistic HER to achieve the activity of the precious metal platinum.
2.2 MXene hybrid/composite materials for electrocatalytic OER and water splitting
Compared with HER, the OER reaction is more complicated because it involves a four-electron transfer process and multiple reaction intermediates. Achieving efficient and durable OER usually requires electrocatalysts with complex heterogeneous structures and designs. Although pure MXenes are not efficient OER catalysts, they can be used as conductive carriers to adjust the electron density of the OER promoters they are coupled to, and even generate additional OER active sites to greatly enhance OER activity. Numerous OER active materials are used to compound with MXenes to prepare hybrid materials with high OER activity, including transition metal sulfides, oxides, nitrides and phosphides, as well as MOFs, LDHs, carbon-based materials and quantum dots. More importantly, the MXene hybrid materials prepared by these strategies are even more active than the noble metal Pt catalysts in point electrocatalytic OER.
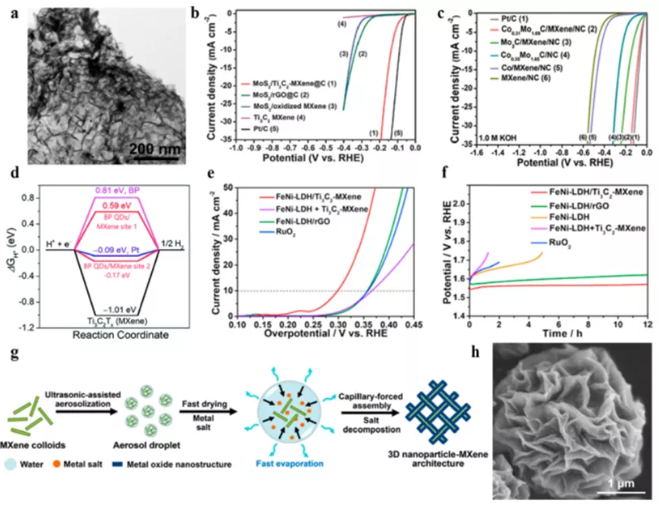
Figure 6 MXene hybrid materials are used for electrocatalytic water splitting. (a) TEM image and (b) MoS2/Ti3C2Tx-MXene@C polarization curve; (c) CoxMo2−xC/MXene/NC polarization curve; (d) BP QDs/Ti3C2Tx free energy diagram; (e ) The polarization curve and (f) constant current stability test of FeNi-LDH/Ti3C2TX-MXene; (g) the assembly process and SEM image of 3DCoP-MXene.
2.3 MXene hybrid/composite materials for photocatalysis and photocatalytic water splitting
In view of the close matching between the Fermi energy (EF) position of MXenes and the H+/H2 reduction potential, efficient H+ adsorption, and the excellent electrocatalytic water splitting performance as described above, the MXene hybrid material is a photocatalytic/photocatalytic water A reasonable choice for cracking to obtain sunlight as a driving source of renewable energy. Unlike electrocatalysis, photocatalytic reactions require semiconductor light absorbers to generate electron-hole pairs. Since most of MXene is metallic rather than semiconducting, other materials must be used to collect light and promote the separation of photogenerated carriers. Some work reported that MXene hybrids are used as multifunctional photocatalysis, HER, OER and water. Cracking catalyst.
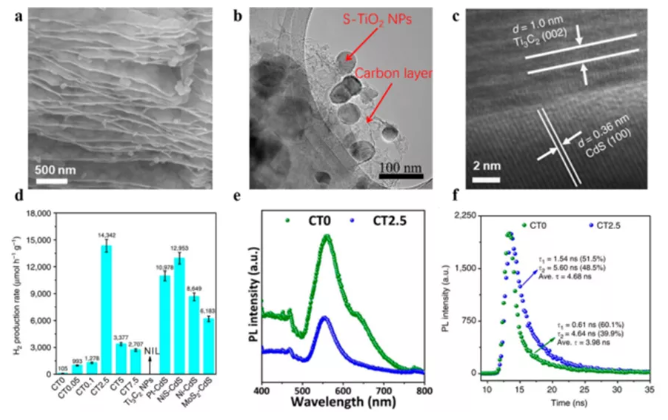
Figure 7 MXene hybrid material is used for photocatalysis HER. (A) Ti3C2Tx derived 2D layered C/TiO2 scan image; (b) Ti3C2Tx derived S-TiO2/C high resolution transmission electron microscope (HRTEM); (c) 2.5%-Ti3C2Tx: HRTEM image of cadmium sulfide mixture; (d) ) Photocatalytic HER activity of various promoters hybridized with cadmium sulfide; PL spectra and time-resolved PL spectra of (ef) CdS and (CT0) and CT2.5 MXene hybrids.
2.4 Chapter summary
In this section, different materials are mixed with MXenes to prepare hybrid materials for electrocatalysis/photocatalysis/photocatalysis, HER, OER and water splitting. In general, MXenes plays various key roles:
(1) Provide large-area conduction and structural support; (2) Act as a HER promoter; (3) Limit, stabilize and prevent the aggregation of hybrid materials; (4) Induce charge transfer to adjust the electronic band structure of hybrid materials , So as to optimize H+, OH- and water adsorption kinetics when chemically coupling with other materials.
3. MXene hybrid materials for metal-air batteries and metal-sulfur batteries
In addition to high HER and OER catalytic activity for water splitting, MXene hybrid materials are also effective in catalyzing OER/ORR redox in metal-air batteries. MXene hybrid has high metal conductivity. Therefore, in lithium-sulfur batteries, MXene hybrid materials can also promote the redox reaction electron transfer of sulfur cathodes. MXene hybrid materials have recently been proven to have high binding affinity to lithium polysulfide, which can extend the cycle life of lithium-sulfur batteries and reduce capacity degradation.
3.1 Application in metal-air battery
The theoretical energy density of metal-air batteries is far from being achieved because of their poor air cathode performance. The complex, multi-step, four-electron OER/ORR redox pair is severely hampered by the slow kinetics as a rate determining step. Although MXenes are not OER- or ORR-active as mentioned earlier, MXenes can be used as conductive supports for other ORR-active electrocatalysts. Mixing MXenes with other materials can adjust the electronic structure and generate catalytic units to enhance the performance of OER, ORR or dual-function OER/ORR.
MXene hybrid material-based metal-air battery is still in the early development stage, mainly based on Ti3C2Tx zinc-air battery. Theoretically, because MXenes can adsorb lithium species and realize interfacial charge transfer and redox reactions, it is expected that the MXene-derived catalyst carrier of lithium-air batteries has high efficiency. Therefore, it is desirable to use other highly conductive MXenes other than Ti3C2Tx to develop MXene hybrid materials for other metal-air chemistry, such as lithium, sodium, magnesium, and aluminum.
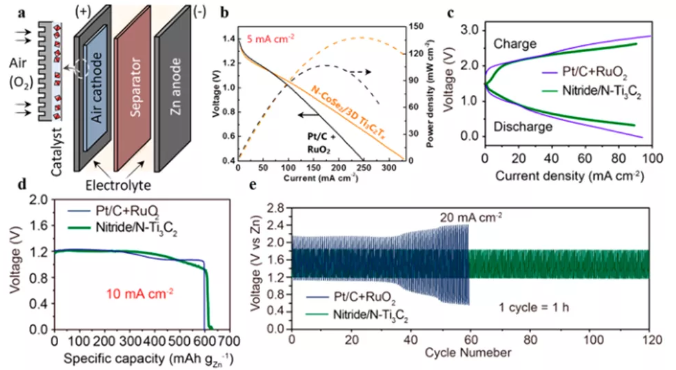
Figure 8 (a) MXene hybrid material as the anode of zinc-air battery; (b) discharge and power density curve; (c) charge and discharge polarization curve; (d) specific capacity; (e) constant current charge and discharge cycle.
3.2 Application in metal-sulfur batteries
Due to the combination of metal conductivity and polar Tx groups, MXenes have been used as sulfur cathodes and separator barriers because they can chemically interact with LiPSs and promote electron transport inside the anode. Experimentally, high conductivity MXenes has been studied as a cathode material. Theoretical research also revealed a variety of MXene junctions
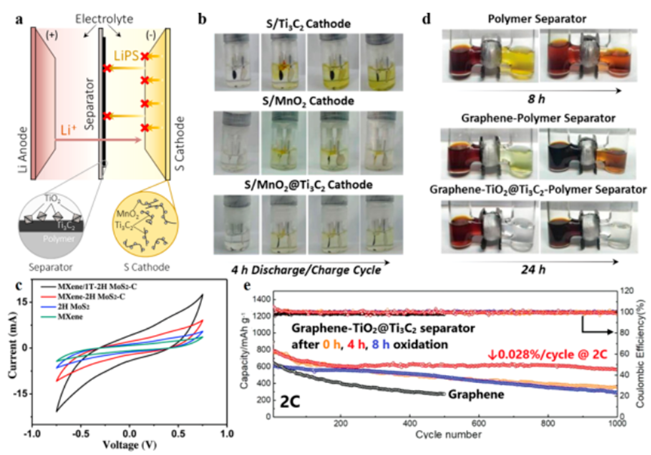
Figure 9 is the MXene hybrid material for the positive electrode and separator of a lithium-sulfur battery. (A) Lithium-sulfur battery; (b) S/Mn3O4@Ti3C2Tx sulfur cathode charge and discharge; (c) Cyclic voltammetry curve of symmetrical battery; (d) LiPSs penetration test; (e) Long cycle performance.
4. Other applications of MXene hybrid materials
4.1 CO2 reduction
So far, the synthesized MXenes are similar to the non-MXene transition metal carbides and nitrides of the bulk 3D bulk in terms of high electronic conductivity. In addition, at room temperature or in an electrolyte environment, Tx groups exist on the basal surface of MXenes. These Tx groups usually contain electronegative elements (fluorine, oxygen, chlorine), which can change the effective work function of MXenes and generate unique interactions between MXene and intermediate molecular adsorbates. In the CO2 reduction reaction, these unusual interactions provide a thermodynamic and kinetic approach to achieve reactions that were not previously possible on transition metal catalysts. In addition to providing active sites for CO2 reduction reaction intermediates, Chen et al. reported the possibility of the Tx group of MXenes participating in the catalytic process.
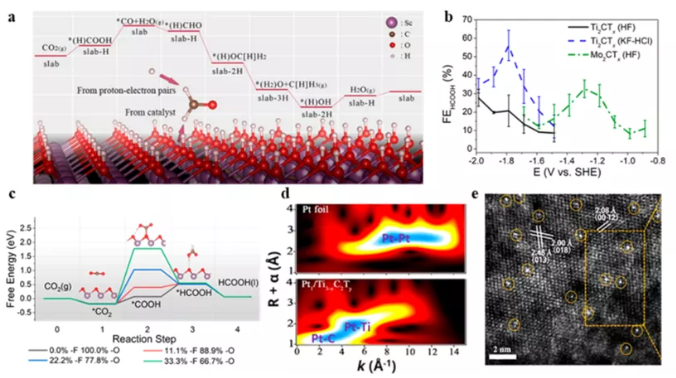
Figure 10 Application of MXenes in the field of CO2 reduction reactions. (A) DFT calculation; (b) Faraday efficiency of CO2 reduction on Mo2CTx and Mo2CTx; (c) calculated free energy diagram. (d) Two-dimensional wavelet transform; (e) HAADF STEM image.
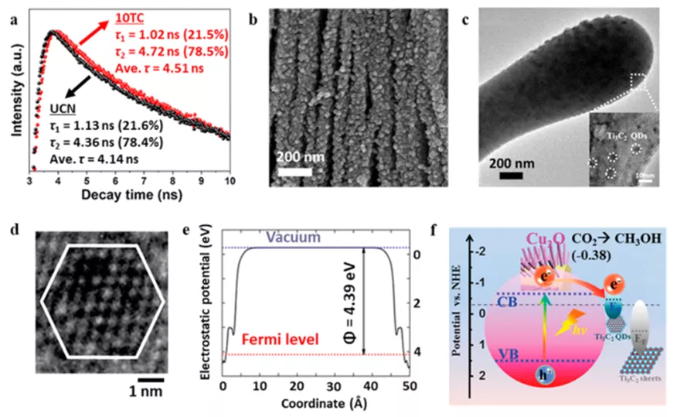
Figure 11 MXene hybrid materials are used for electrocatalysis and photoelectrocatalysis for CO2 reduction. (A) Transient PL spectrum; (b) Ti3C2Tx/TiO2 morphology; (c) Ti3C2Tx quantum dot/Cu2O transmission image and (d) Ti3C2Tx quantum dot HRTEM image; (e) DFT calculation of Fermi level and ( f) Energy band diagram.
4.2 Nitrogen reduction
Electrocatalytic/photocatalytic nitrogen reduction provides a more attractive alternative to the traditional Haber-Bosch process. The reduction of carbon dioxide can be carried out at room temperature, which is a potentially safer, cleaner, and more sustainable method of producing NH3. Combined with sustainable energy input, the factory has a smaller footprint. The MXenes that are more favorable for N2 adsorption on the base surface include pure M2CTx, M3C2Tx containing group 14 "M", and ordered double transition metal Mo2TiC2Tx. Conversely, remote adsorption is more conducive to the occurrence of M3C2T containing 15 and 16 group "M". However, it should be noted that pure MXenes cannot be stable under N2 reduction reaction conditions, and the Tx group contained in it needs to be considered in the theoretical calculation. Although alternative adsorption sites can be provided on the exposed "M" sites or monoatomic dopants at the edges, the Tx group usually makes N2 adsorption on MXenes more difficult.
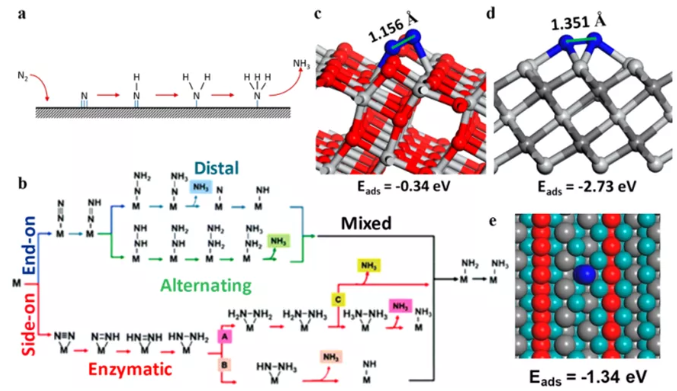
Figure 12 Study on the mechanism of N2 reduction reaction. (A) Dissociated N2 is reduced to NH3; (b) Combined N2 is reduced to NH3; schematic diagram of N2 adsorption on the (c) TiO2 (101) surface and (d) Ti3C2TxMXene side; (e) Ti exposed on the edge of Ti3C2O2 MXene The site undergoes endpoint N2 adsorption.
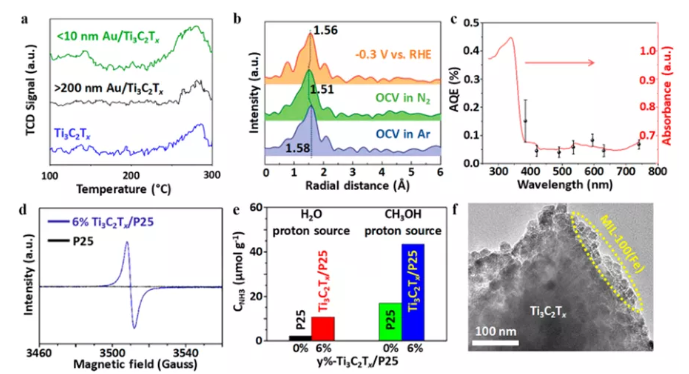
Figure 13 MXene hybrid material for N2 reduction reaction. (A) N2 temperature programmed desorption curve; (b) FT-EXAFS spectrum; (c) N2 fixed AQEs on Ti3C2Tx/TiO2; (d) ESR spectrum; (e) NH3 yield; (f) 15%-Ti3C2Tx /MIL-100(Fe) HRTEM image of hybrid material.
[Conclusions and Prospects]
In this review, the author discusses the design principles of MXene hybrid materials in catalytic energy storage and conversion applications. The hybridization and synthesis methods of different kinds of second phase materials and MXenes are summarized, and their differences are highlighted. In future research, efforts should be made to develop a gentle and scalable top-down synthesis and hybridization method of MXene to achieve the following goals: (1) Minimize the oxidation and mechanical loss of MXene during processing, especially at high temperatures; (2) It can better control the chemical composition, morphology and distribution of the second phase material grown on MXene; (3) Realize a highly porous 3D MXene hybrid network to achieve more efficient quality and long-range charge transfer, thereby further Enhance catalytic activity. In view of the broad research prospects of MXene hybrid materials and composite materials, it is expected that experimental and theoretical work on the development of MXene hybrid materials for clean energy and related applications will increase in the near future.
Kang Rui Garrick Lim, Albertus D. Handoko, Srinivasa Kartik Nemani, Brian Wyatt, Hai-Ying Jiang, Junwang Tang, Babak Anasori, and Zhi Wei Seh, Rational Design of Two-Dimensional Transition Metal Carbide/Nitride (MXene) Hybrids and Nanocomposites for Catalytic Energy Storage and Conversion, ACS Nano, 2020, DOI:10.1021/acsnano.0c05482
Article source: Energy scholar
This information comes from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately.
- Previous: ESM: The influence of
- Next: MXene breakthrough: Na


 mxene academic
mxene academic