ACS Nano: Ru/MXene/TiO2 co-catalyst improves photocatalytic hydrogen production performance
QQ Academic Group: 1092348845
Detailed

South China University of Technology Peng Feng, Peng Xinwen, etc. reported the synthesis of restricted Ti3C2/Ru co-catalysts. The synthesis method directly reduces Ru3+ without adding a reducing agent. The in-situ formed TiO2 nanosheets are modified on the surface of Ti3C2/Ru. , While maintaining a good separation between the semiconductor TiO2 and Ti3C2/Ru co-catalyst, because the catalyst has a lower Fermi energy level, the self-assembled Ti3C2/Ru co-catalyst can receive photo-generated electrons and improve the process of generating H2. The catalyst exhibits high stability. The author used DFT calculations and in-situ DRIFT infrared spectroscopy to test the interface work function changes, and found that the Ti3C2/Ru photocatalysts with separated structures effectively improved the charge transfer efficiency and the photocatalytic H2 generation efficiency. This work provides design guidance experience for the development of semiconductor co-catalysts for solar energy conversion catalysts.
Highlights of this article
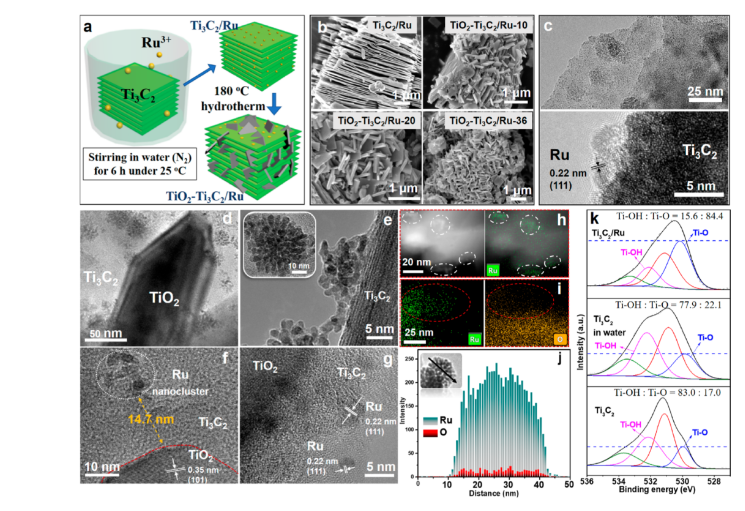
Point 1. Catalyst synthesis. Ti3C2 was obtained by peeling off Ti3AlC2, then ultrasonic treatment in N2 environment, adding RuCl3 and vacuum drying treatment at 80 ℃ in N2 atmosphere for 12 h to obtain Ti3C2/Ru.
Disperse Ti3C2/Ru and NaBF4 in 1 M HCl solution, ultrasonically treat for 20 min, stir for 10 min, then hydrothermally heat at 180 ℃ for 10 to 36 h, and then dry at 70 ℃ in vacuum for 12 h to obtain TiO2-Ti3C2 /Ru.
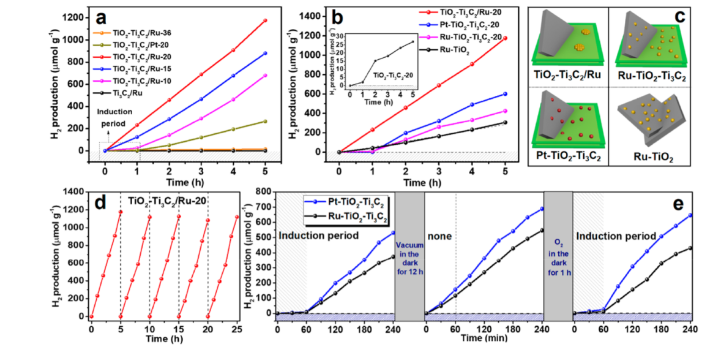
Point 2. In this reaction, there is no need to add a reducing agent during the reduction of Ru3+ to Ru0 on the Ti3C2 interface, resulting in a higher interaction between Ru and Ti3C2. The photochemical analysis results verify the ratio of separated TiO2-Ti3C2/Ru catalysts. The combined Ru-TiO2-Ti3C2 catalyst has more excellent charge separation activity; the work function of Ti3C2 in the Ru-TiO2-Ti3C2 catalyst is improved, resulting in Ti3C2 instead of Ru as the electron acceptor, and a large number of OH functional groups are formed on the Ti3C2 interface during the light irradiation , Resulting in a decrease in work function, which enables effective photocatalytic reactions. In contrast, in the TiO2-Ti3C2/Ru catalyst, electrons and holes are well separated and exhibit higher activity in photocatalytic reactions.
This article shows that Ti3C2 acts as a good co-catalyst for MXene materials.
Article source: Catalytic Meter
- Previous: Nano Letters: MXene-de
- Next: MXene breakthrough: Na


 mxene academic
mxene academic
