AFM: V-based MXene in situ electrochemical activation for zinc ion batteries
QQ Academic Group: 1092348845
Detailed


In recent years, due to the high discharge capacity generated by the multi-electron redox chemical reaction of vanadium, vanadium-based materials with abundant composition and crystal structure have attracted great interest. V2C MXene has ultra-high electrical conductivity and rich surface chemistry, which provides a huge opportunity for building such high-performance ZIB. The accordion-like V2CTX is composed of a large number of V-C-V single-layer stacks, showing many ordered nano-channels to promote charge transfer. It can be used as a multi-branch parallel circuit to provide high power output. In addition, the accessible surface of 2D V2CTX provides sufficient active sites for the intercalation/deintercalation of Zn2+ ions. However, to date, there are almost no reports on the use of V2CTx as a ZIB positive electrode because V2CTx exhibits an unreasonably low discharge capacity. We believe that the poor redox activity of V2CTx is mainly due to its low valence V atoms (+2 and +3), which cannot participate in the multi-electron redox reaction through the high and low valence transitions during the charge/discharge process. Taking into account the common multi-electron transfer potential of V, the Zn ion storage capacity of V2CTx can be improved by increasing the valence state of its V atom. Even better, if the conductive V-C-V 2D structure of V2CTX is intentionally retained, theoretically, high-rate Zn ion storage capacity can be achieved.

Recently, Dr. Yang Huang and Wang Zhang of Shenzhen University, Shenzhen University, and Professor Shu Lei of the University of Wollongong, Australia, published the topic In-Situ Electrochemically Activated Surface Vanadium Valence in V2C MXene to Achieve High Capacity and Superior in the internationally renowned academic journal Advanced Functional Materials. Research paper on Rate Performance for Zn-Ion Batteries. In this study, the V2CTx anode was electrochemically activated in situ by initial charging at a certain potential. This strategy can adjust the valence of surface V atoms without disturbing the internal conductive V-C-V 2D structure. The activated V2CTx positive electrode showed a high capacity of 423.5 mAh g-1 at 1 A g-1, and it was as high as 358 mAh g-1 even at a current density of 30 A g-1. Studies have shown that surface V atoms undergo electrochemical oxidation during the initial charging process and are converted to VOx, causing the valence of V to rise significantly from V2+ / V3+ to V4+ /V5+. At the same time, by accurately controlling the charging voltage (for example, 1.8 V) and the holding time (for example, 2 h), the internal conductive V-C-V multilayer structure can be well preserved. In view of the structural advantages of the design, the activated V2CTx also has excellent long-term cycle performance, showing a discharge capacity of 283.7 mAh g-1 after 2000 cycles. This work opens up an effective way for the rational design of electrode materials for water-based ZIBs with high energy and power density.

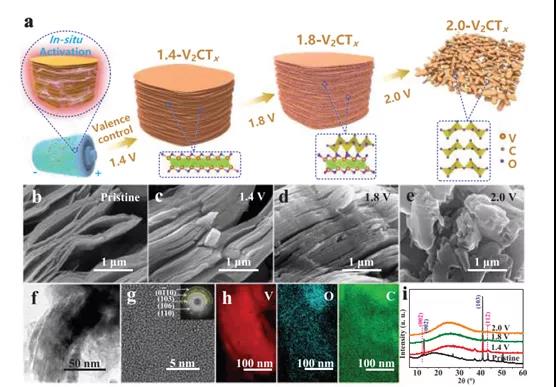
Figure 1. Schematic diagram, morphology and structure evolution of V2CTx anodes with different activation voltages
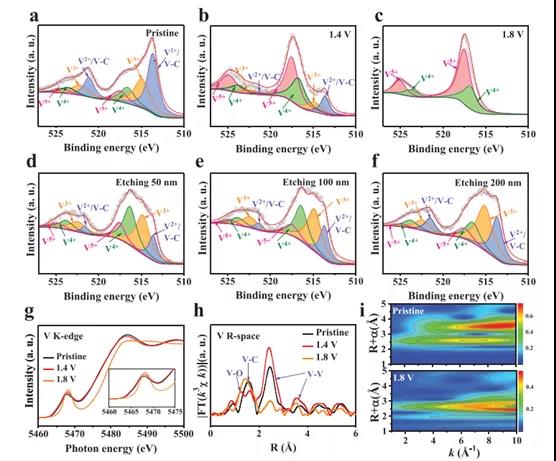
Figure 2. The chemical state and electronic structure of V2CTx anodes with different activation voltages.
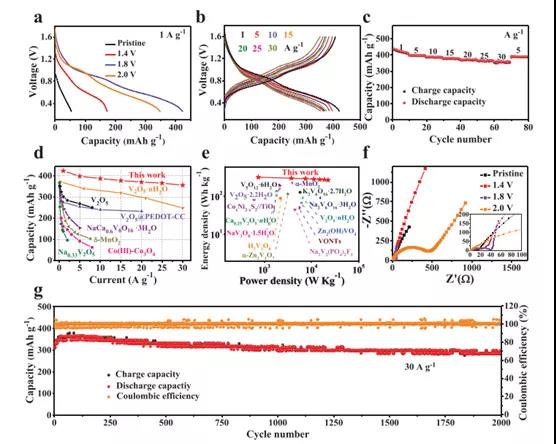
Figure 3. Electrochemical performance of V2CTx cathodes with different activation voltages in Zn ion storage
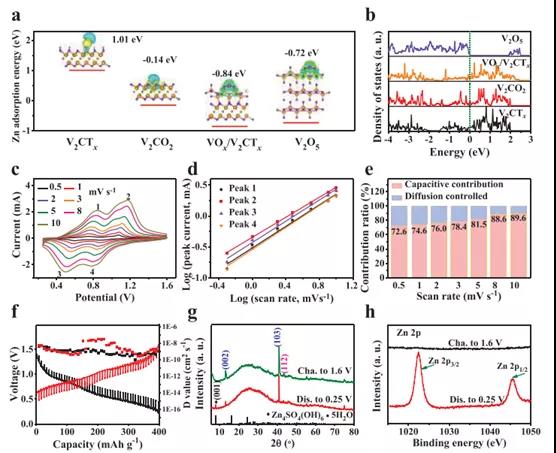
Figure 4. DFT calculation results, kinetic analysis and characterization of V2CTx cathode

By adjusting the valence state of vanadium on the surface of V2CTx through in-situ electrochemical activation, high-capacity and high-rate Zn ion storage performance has been successfully achieved. Due to the reforming of surface V atoms, high-valence V appears in the activated V2CTx positive electrode, which leads to a significant increase in Zn ion storage capacity. At the same time, the internal V–C–V multilayer structure of V2CTx is intentionally retained, providing a rich and ordered nanochannel with essentially high electronic/ion conductivity for rapid electrochemical reactions. Therefore, the best 1.8-V2CTx can achieve an excellent rate performance of 358 mAh g-1 even at 30A g-1. The detailed kinetic analysis of the activated V2CTx anode shows that the charge storage mechanism is mainly dominated by capacitive behavior, with a significantly high diffusion coefficient Zn2+ ≈10-7 -10-10 cm2 s-1. This strongly proves the structural advantages of the synergistic effect between the external high-priced VOx and the internal conductive V-C-V multilayer of the V2CTx positive electrode. This discovery will promote the development of MXene cathodes through appropriate surface modification, thereby giving full play to its potential in high-performance ZIB.
Literature link:
DOI: 10.1002/adfm.202008033
Information source: MXene Frontier
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
- Previous: JMCA: 3D high-density
- Next: MXene breakthrough: Na


 mxene academic
mxene academic
