Study on photocatalytic nitrogen fixation of MXene/TiO2 composite catalyst modified by Luo Min team
QQ Academic Group: 1092348845
Detailed
Recently, Gao Wanguo, a 2018-level graduate student of chemical engineering in the research group, published a paper in the journal "Journal of Colloid and Interface Science" (JCR District 1, Impact Factor 2020: 7.489) under Elsevier. Thesis: "In situ modification of cobalt on MXene / TiO2 as composite photocatalyst for efficient nitrogen fixation"; DOI: 10.1016 / j.jcis.2020.11.064, first author: High nations, Corresponding author: Man Li, Associate Professor, Professor Luo Min .

Full text at a glance
In this work, anatase TiO2 was grown in situ on the surface of MXene by calcination method using MXene as the precursor, and Co doping was successfully introduced by impregnation method, and finally the MXene/TiO2/Co ternary composite catalyst was successfully prepared. The catalyst has high efficiency and stable performance of photocatalytic nitrogen fixation to synthesize ammonia. The experimental results show that the excellent catalytic performance is due to the heterojunction formed by MXene and in-situ TiO2, which accelerates the separation and migration of photogenerated carriers; in addition, combined with DFT theoretical calculations, the transition metal Co can effectively regulate the catalyst. The active site balances the adsorption and desorption of the reactant (N2) and the product (NH3), so that the reactant desorbs from the active site in time, which promotes the utilization efficiency of the active site and improves the nitrogen fixation performance.
Background introduction
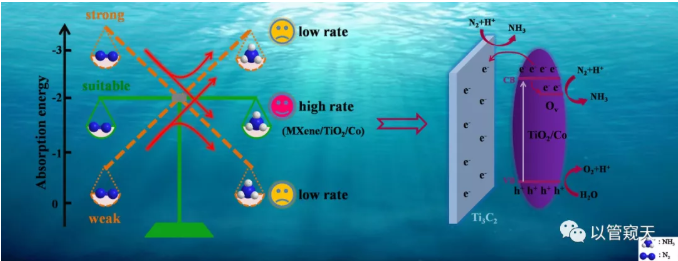
As an important intermediate in the global nitrogen cycle, ammonia is widely used in the fields of synthetic chemicals, pharmaceuticals, fertilizers, and energy storage. At present, synthetic ammonia mainly relies on the "Haber-Bosch" reaction to achieve, but the technical reaction conditions are harsh, not only consumes huge energy, but also emits a large amount of greenhouse gas CO2. In recent years, photocatalytic nitrogen fixation to synthesize ammonia (in the water phase with solar energy as the driving force, water as the proton source, reaction under normal temperature and pressure) has attracted widespread attention.
Experimental design ideas
The current photocatalytic nitrogen fixation efficiency is far from the practical application level, mainly due to the serious recombination of photogenerated carriers and the low efficiency of active sites. In view of the photo-generated carrier recombination problem,the formation of a tightly bound heterojunction in situ can effectively accelerate the carrier separation and migration efficiency; in view of the low efficiency of the active site,
Graphic analysis
1. Characterization of material structure morphology
XRD, DRS and XPS results show that Co doping promotes the conversion of surface MXene to anatase TiO2; XPS results show that Co exists in the form of Co2+, and the incorporation of its valence state leads to the formation of a larger proportion of O vacancies; SEM, HRTEM The results of Mapping and Mapping show that MXene and anatase TiO2 coexist in the material, and prove that Co is highly dispersed in the catalyst.
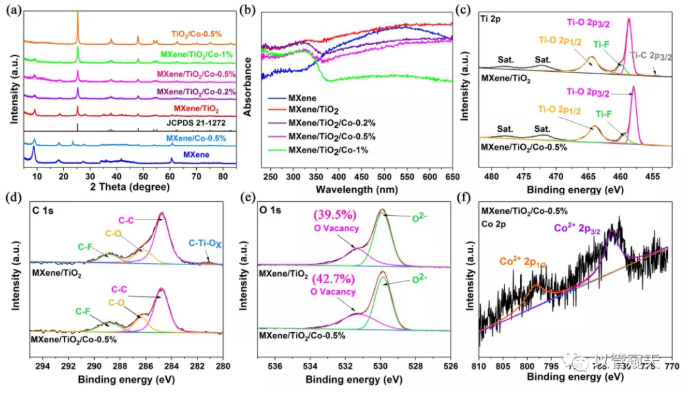
Figure 1. (a) X-ray diffraction (XRD), (b) UV-vis diffuse reflectance spectra (UV-vis DRS) of the prepared samples, (c) Ti 2p, (d) C 1s and (e) O1s XPS spectra of MXene/TiO2 and MXene/TiO2/Co-0.5%, (f) Co 2p XPS spectra of MXene/TiO2/Co-0.5%.
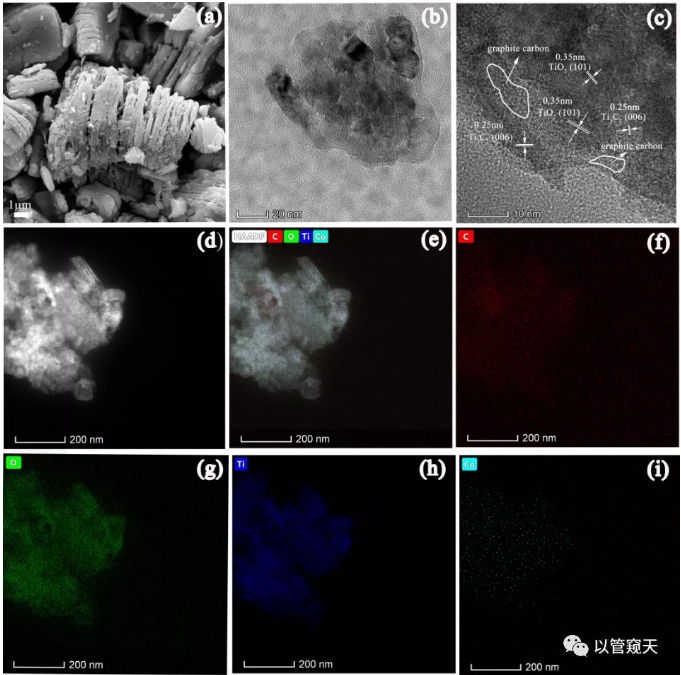
Figure 2. (a) scanning electron microscopy (SEM), (b) transmission electron microscopy (TEM), (c) high-resolution transmission electron microscopy (HRTEM), (d) HAADF, and (ei) EDS mapping of MXene/ TiO2/Co-0.5%, (f) C, (g) O, (h) Ti (i) Co.
2. Catalytic performance test
The performance of MXene/TiO2 in the binary system is better than that of the unary systems MXene and TiO2. This is because the formation of the heterojunction accelerates the separation and migration of carriers and improves the nitrogen fixation performance. In the binary system, MXene/Co and TiO2/Co The catalytic performance is better than that of unary MXene and TiO2, respectively. It is speculated that this is because Co modification adjusts the balance of adsorption and desorption of reactants and products, and promotes nitrogen fixation performance. The final ternary system exhibited superior nitrogen fixation performance under the combined effect of heterojunction and Co modification. Among them, 0.5% Co doped samples performed best. Control experiments show that there is no impurity interference in the reaction process. Cycle experiments and yield over time show that the catalyst is stable.
Figure 3. (a) NH4+ production rate under air ambient, (b) NH4+ production rate of the control experiments in Ar, Air, N2, and dark ambient, (c) the stability of N2 photofixation activity of MXene/TiO2/Co- 0.5% under N2 and Air atmosphere, (d) NH4+ production rate of MXene/TiO2/Co-0.5% along with the reaction time under N2 and Air atmosphere.
3. Mechanism discussion
Figure 4 shows that the formation of heterojunction accelerates the separation and transfer of photogenerated carriers through EIS and photocurrent response; Figure 5 EPR results confirm that the incorporation of Co introduces more oxygen vacancies and makes the material have more active sites The results of N2-TPD and NH3-TPD prove that the incorporation of Co significantly adjusts the chemical adsorption of the reactant (N2) and the product (NH3) by the catalyst; Figure 6 DFT calculation results show that the incorporation of transition metals can simultaneously affect the material to N2 and NH3 The adsorption energy of N2 and NH3 is adjusted by the doping of Co to adjust the adsorption energy of moderate and balanced state.
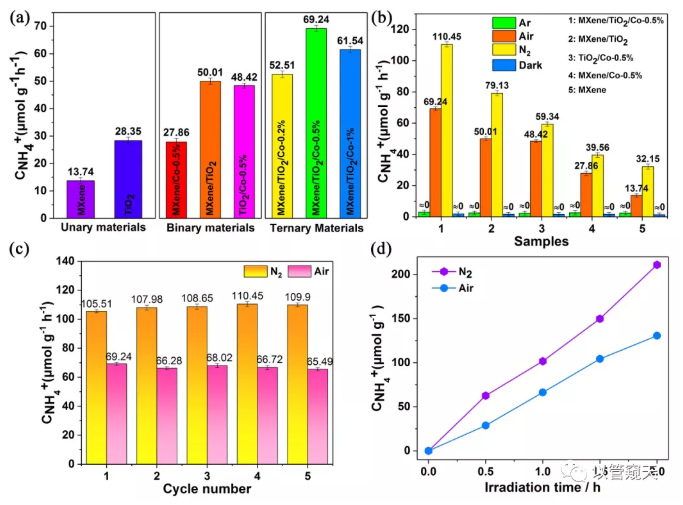
Figure 4. (a) Electrochemical impedance spectra EIS of MXene and MXene/TiO2/Co-0.5% under dark and light conditions, (b) Photocurrent spectra of MXene, MXene/TiO2 and MXene/TiO2/Co-0.5%.
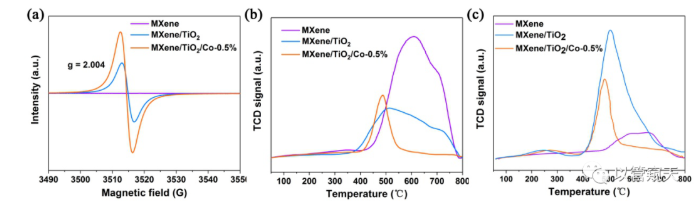
Figure 5. (a) Electron paramagnetic resonance (EPR) spectra, (b) N2-TPD, and (c) NH3-TPD spectra of MXene, MXene/TiO2 and MXene/TiO2/Co-0.5%, respectively.
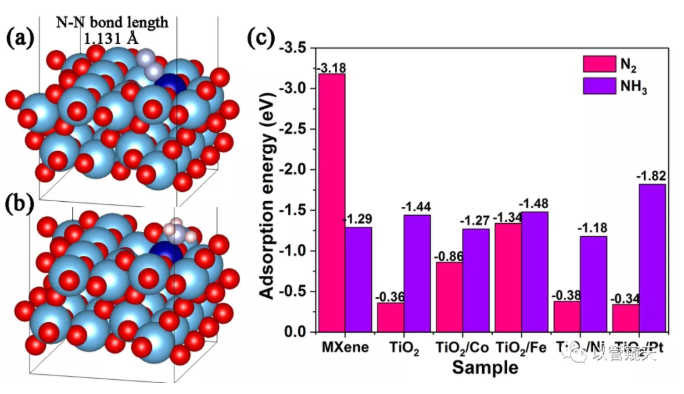
Figure 6. Schematic adsorption model for (a) N2 and (b) NH3 on the surface of TiO2/Co, (c) Adsorption energy of N2 and NH3 on MXene, TiO2, TiO2/Co, TiO2/Fe, TiO2/Ni, and TiO2/Pt.
Summary and outlook
In summary, the in-situ growth of MXene/TiO2 heterojunction by calcination improves the transfer and separation of the carrier, and Co doping effectively adjusts the chemical adsorption balance of reactant N2 and product NH3 on the catalyst surface. Finally, high-efficiency and stable photocatalytic nitrogen fixation is realized. Under N2 environment and UV-visible light, without adding any pore sacrificial agent, the yield of ammonia in pure water can be as high as 110 μmol g-1h-1. Previous reports on photocatalytic nitrogen fixation mainly focused on the strong chemisorption of the reactant N2 by the catalyst, which is beneficial to weaken and activate N2. This work not only adjusted the adsorption energy of the reactants on the catalyst surface, but also proposed for the first time the influence of the product NH3 adsorption energy on the catalytic performance, and improved the catalytic performance by improving the product desorption. We advocate that while studying the adsorption energy of catalysts on reactants, we should also pay attention to the adsorption energy of catalysts on products.
Introduction of the research group and corresponding author
Luo Min: Professor of School of Chemistry and Chemical Engineering of Ningxia University, Master/PhD supervisor. The leader of the scientific research and innovation
Li Xiaoman: Associate Professor of School of Chemistry and Chemical Engineering, Ningxia University, master supervisor. In 2017, he graduated from the Shanghai Institute of Ceramics, Chinese Academy of Sciences with a Ph.D. Research directions are low-dimensional nanomaterials, photoelectric catalysis, nitrogen reduction, and carbon dioxide reduction. Published more than 10 SCI papers in foreign academic journals.
Information source: from a limited perspective
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
- Previous: ACS AMI: Mo-based MXen
- Next: MXene breakthrough: Na


 mxene academic
mxene academic
