One shot is 2 Nature Nanotechnology! This biomaterials big cow understands
QQ Academic Group: 1092348845
Detailed
Infection is the main factor contributing to the global burden of disease. The high mortality rate is related to diseases such as lower respiratory tract infections, diarrhea, tuberculosis, human immunodeficiency virus infection and malaria. The mortality rate is highest in developing countries, and resources such as vaccines and anti-infectives may be limited in these areas. In addition, clinical trials of infectious diseases (IDs) are also behind, so there is an urgent need to determine practical and effective strategies that can better treat infectious diseases.
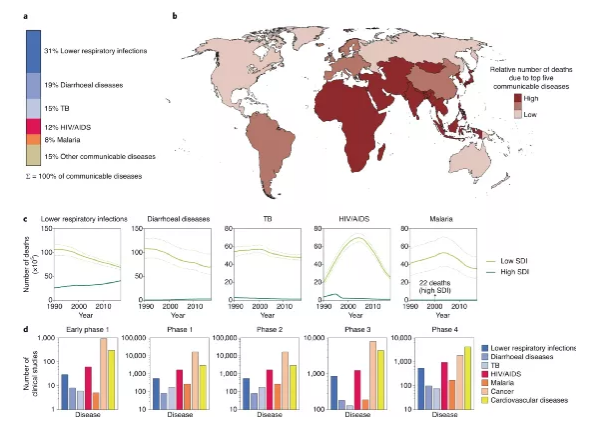
Figure | Global Burden of Infectious Diseases
To effectively manage infectious diseases, many challenges must be overcome. For example, lack of safe and effective drugs, poor procurement practices, inability to pay for drugs, and poor stability of drugs under high temperature and high humidity. To overcome these challenges, we must make concerted efforts at the scientific and policy levels.
Nanotechnology has the potential to change the detection and treatment of many diseases. In the past few decades, nanosystems have been the subject of intensive research, resulting in FDA-approved chemotherapeutics, anesthetics, imaging agents, nutritional supplements, etc. Not surprisingly, nanotechnology has been widely evaluated to improve the treatment of ID.
In view of this, MIT Giovanni Traverso and others (of whom Academician Robert Langer is one of the authors) discussed the opportunities provided by nanotechnology for the treatment of IDs on Nature Nanotechnology, and discussed the preclinical and clinical progress in this field. The focus is on HIV, TB and malaria. In addition, the researchers also recognized that nanotechnology is being successfully applied to SARS-CoV-2 mRNA and protein vaccines. Finally, they discussed the challenges of applying these techniques from the laboratory to the clinic.
The availability and correct use of safe and effective drugs for the treatment of infectious diseases by nanotechnology are necessary conditions for the treatment of IDs. Nanotechnology-based methods have become the subject of intensive preclinical evaluation to increase the therapeutic index of ID drugs and simplify their use. Researchers focus on promising preclinical research and discuss the challenges of its clinical translation.
Continuous systemic administration of anti-infectious drugs The administration of many infectious diseases involves long-term treatment, which places a heavy burden on patients and the healthcare system. For example, HIV and tuberculosis, which lead to lower patient compliance, and ultimately lead to treatment failure. Therefore, the development of a system that reduces the frequency of doses and simplifies the dosing schedule has attracted widespread interest.
At present, scientists have been actively seeking injectable nanocarriers that can continuously deliver drugs. Broadly speaking, these systems can be divided into two categories: systems that control drug release through excipients (such as polymers or lipids), and systems that rely on poorly soluble drug crystals to slowly dissolve in the tissue fluid.
Quite a lot of research has focused on polyester-based and liposomal drug delivery systems. The disadvantage of polymer and liposome formulations is that they require a large amount of excipients to control drug release. This increases the volume of the injection and limits the dosage of the drug. An important consideration for sustained-release systems is the possibility of long-term exposure to sub-therapeutic concentrations, which may lead to drug resistance.

Figure | Nanocarriers for continuous local and systemic drug delivery
Local administration As the epithelial surface is a common site for pathogens to enter and reside, regional drug delivery to these surfaces has broad prospects. Compared with systemic administration, local delivery to the site of infection may increase (and minimize off-target) drug exposure. The current research involves: a vaginal local drug delivery system that manages to quickly pass through the mucosa, pulmonary delivery to treat respiratory infections, and topical topical treatment of wound infections.
Targeted delivery to the site of infection has aroused widespread research interest in the design of systems that can deliver drugs to the site of infection in a targeted manner. This is due to the low penetration rate of the drug into the infected tissue (for example, the low penetration rate of antiretroviral drugs to the brain and lymph nodes and the low penetration rate of anti-tuberculosis drugs to cavity lesions), the distribution of the drug into the toxic site and the symbiotic microorganisms Caused by killing. Encapsulation in nanocarriers provides the possibility of drug targeting, but there is still much room for improvement. Compared with uninfected tissues, due to the enhanced permeability of nanocarriers at the infected site, better targeted aggregation occurs. The surface of the nanocarrier can also be functionalized with ligands that bind to infected tissues or microorganisms. The latter strategy is called active targeting or ligand-mediated targeting. Therefore, strategies that do not rely on specific ligands are called passive targeting.
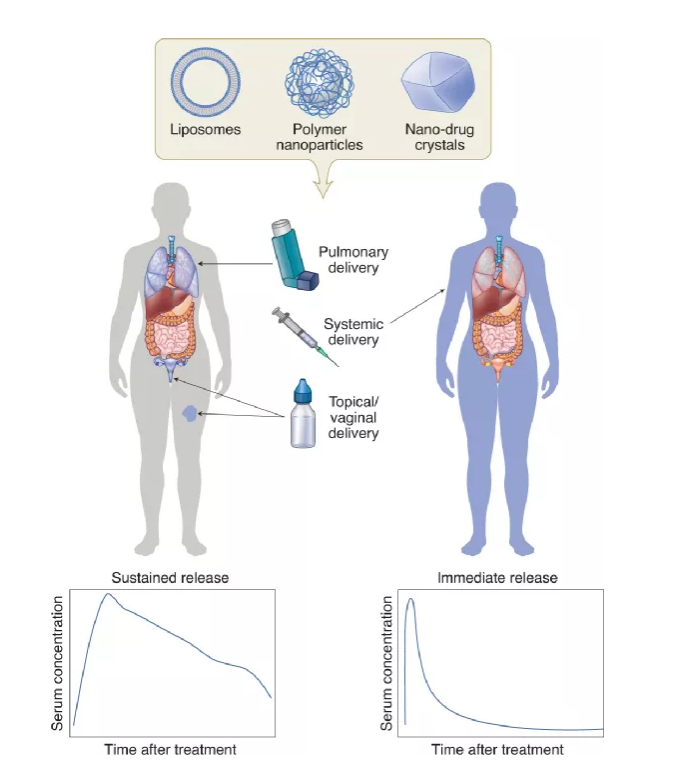
Figure | Nanocarriers for targeted drug delivery
Overcoming resistance to anti-infectives Overcoming resistance is a key and growing obstacle to the treatment of IDs. Drug resistance is caused by the secretion of drug-inactivating enzymes and the transformation of pathogens into a state of reduced metabolic activity, the formation of biofilms, the use of intracellular life cycles, or obligate intracellular pathogens.
Drugs spread poorly on the host cell membrane, and active efflux through drug transporters can reduce the activity against intracellular pathogens. Another complication is that drug molecules and pathogens are unevenly distributed in the host cell, and without co-localization, the efficacy is affected. For example, aminoglycosides accumulate in large amounts in lysosomes and may be ineffective against cytoplasmic pathogens. Therefore, strategies to enhance intracellular accumulation of drugs and target subcellular locations may improve the efficacy of anti-infective drugs.
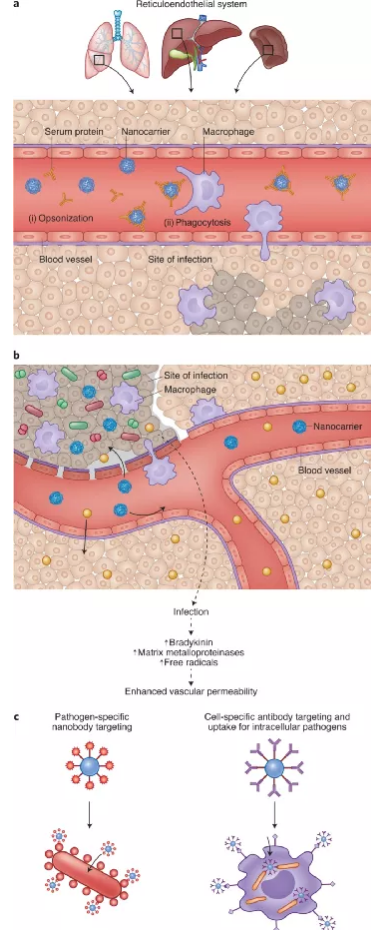
Figure | Nanocarriers overcome drug resistance
Under advanced preclinical and clinical nanotechnology, researchers will focus on the application of the most advanced nanotechnology in HIV infection, tuberculosis and malaria. Readers will learn that nanotechnology has received the most active research in clinical and large animals for the treatment and prevention of HIV infection. In contrast, for malaria and tuberculosis, peoples pursuit of nanotechnology is less enthusiastic. For these IDs, work is mainly limited to preclinical trials in rodents. The researchers also hope to convey that the most advanced nanotechnology is not complicated, but it must have a strong influence. Therefore, perhaps in order for nanotechnology to benefit patients, it needs to meet certain principles, such as simple design, clinical needs, and economic enthusiasm.
AIDS HIV infection is one of the main IDs leading to high global morbidity and mortality. Long-acting injection of antiretroviral drugs is the most clinically advanced nanotechnology in HIV treatment. Long-acting injectable nanoparticles reduce the frequency of dosing and can improve patient compliance. This is widely regarded as a breakthrough intervention. In addition, oral antiretroviral nanoparticles have been developed for use in pediatrics, aiming to replace tablets (difficult to swallow) and alcohol-containing solutions (containing harmful excipients).
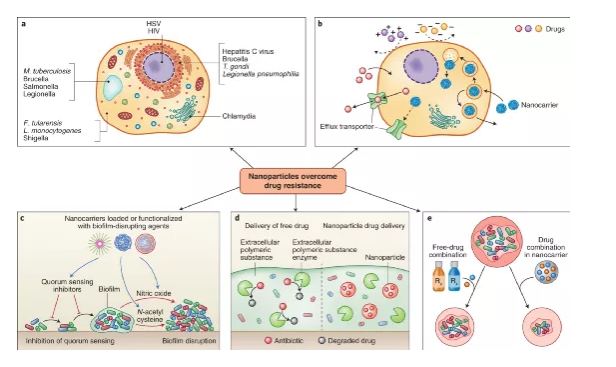
Figure | HIV life cycle
Malaria Malaria caused by a single-celled complex protozoan of the genus Plasmodium is the most prevalent parasitic disease in the world. Although more than 3 billion people are at risk of infection, funding for the global malaria response has been stagnant since 2010, reaching only US$3.1 billion in 2017. As part of the investment, short- and long-term malaria solutions must be considered, such as the use of nanotechnology for treatment and vaccination. Considering that the target product price for the treatment of malaria is US$1 per day, the key consideration for the development of a new method is its cost-effectiveness.
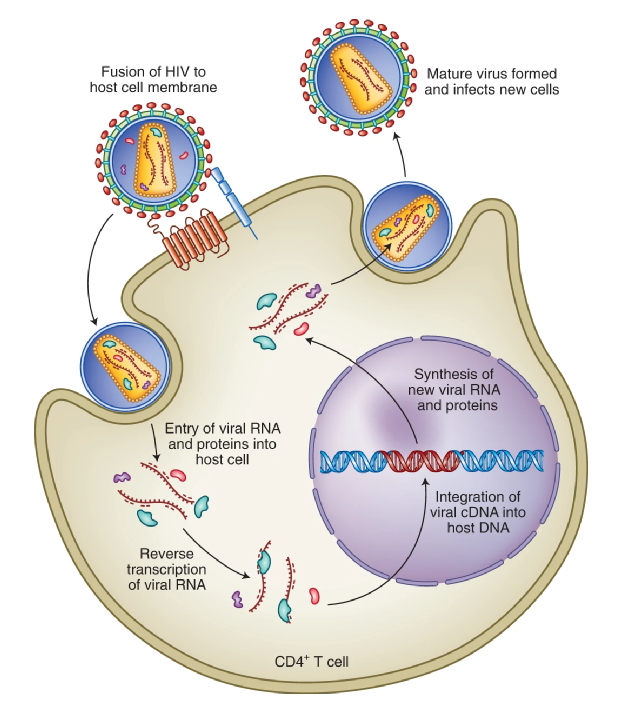
Figure | The life cycle of malaria in two hosts
Tuberculosis Tuberculosis kills more than 3,500 people every day and is the worlds top killer of ID. According to data from the World Health Organization (WHO), 10 million people suffered from tuberculosis in 2017, and the global economic burden reached 12 billion U.S. dollars each year. In addition, Mycobacterium tuberculosis is the most critical pathogen in the global antimicrobial resistance crisis.
WHO guidelines recommend oral medications of four antibiotics per day to treat drug-sensitive tuberculosis for at least 6 months. Due to long-term and frequent dosing and side effects, patients find it difficult to adhere to these treatment regimens and are at risk of developing resistant strains. Therefore, reformulating existing drugs in nanocarriers is an attractive method that has a direct impact on tuberculosis treatment. Several systems based on oral nanotechnology have been designed using existing tuberculosis drugs and tested in animal models. In addition, nanocarriers for inhalable tuberculosis drugs have always been the focus of research. Currently, there is no effective vaccine to prevent tuberculosis infection in an all-round way. Researchers are trying to improve the existing BCG vaccine and derive new vaccines to prevent TB.
Figure | Pathogenesis of tuberculosis infection
Summary and Prospects The above highlights many promising therapeutic strategies for developing nanomedicine to treat and prevent ID. However, in order to have the greatest impact, nanotechnology solutions will need to overcome some economic cost, manufacturing, and regulatory challenges. 1) First, reducing costs will be the main obstacle to the development of new drugs and nanotechnology-based systems. The currently approved nanosystem (an amphotericin B liposome formulation) is not cost-effective in developing countries. 2) Second, the synthesis and storage conditions of some nanoparticles may not be conducive to the conditions of resource-poor countries. 3) Third, in the clinical trial phase, regional and national differences within and between regulatory agencies, especially when conducting multi-center international trials, will be a challenge. 4) Finally, the patient acceptability of these nanosystems needs to be addressed.
In the new crown epidemic, nanotechnology has had a significant impact, which provides broad prospects. Similarly, similar feats of nanotechnology are possible in the treatment of other infectious diseases.
It is worth noting that the research group recently published another research paper on Nature Nanotechnology. They reported on the integration of machine learning and high-throughput experiments to quickly and large-scale identification of such nano-agents. The researchers identified 100 self-assembled drug nanoparticles from 2.1 million pairs, each of which included one of 788 drug candidates and one of 2,686 approved excipients.
Introduction:
Giovanni Traverso is an assistant professor in the Department of Mechanical Engineering at the Massachusetts Institute of Technology (MIT) and a gastroenterologist in the Department of Gastroenterology, Brigham and Womens Hospital, Harvard Medical School. Dr. Traversos previous work focused on the development of new molecular detection methods for the early detection of colon cancer. During his postdoctoral research, he transitioned to the fields of chemistry and biomedical engineering in the laboratory of Professor Robert Langer of the Massachusetts Institute of Technology, where he developed a series of novel technologies for drug delivery and physiology through the gastrointestinal tract Sensing.
His current research plan focuses on developing next-generation drug delivery systems to deliver therapeutic drugs safely and effectively, and developing novel ingestible electronic devices to sense a wide range of physiological and pathophysiological parameters. In addition, Dr. Traverso continues to work on the development of new diagnostic tests that can detect cancer early.
References: 1. Kirtane, AR, Verma, M., Karandikar, P. et al. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. (2021).https://doi.org/10.1038/s41565-021- 00866-82. Reker, D., Rybakova, Y., Kirtane, AR et al. Computationally guided high-throughput design of self-assembling drug nanoparticles. Nat. Nanotechnol. (2021).https://doi.org/10.1038 /s41565-021-00870-y

Figure | Global Burden of Infectious Diseases
To effectively manage infectious diseases, many challenges must be overcome. For example, lack of safe and effective drugs, poor procurement practices, inability to pay for drugs, and poor stability of drugs under high temperature and high humidity. To overcome these challenges, we must make concerted efforts at the scientific and policy levels.
Nanotechnology has the potential to change the detection and treatment of many diseases. In the past few decades, nanosystems have been the subject of intensive research, resulting in FDA-approved chemotherapeutics, anesthetics, imaging agents, nutritional supplements, etc. Not surprisingly, nanotechnology has been widely evaluated to improve the treatment of ID.
In view of this, MIT Giovanni Traverso and others (of whom Academician Robert Langer is one of the authors) discussed the opportunities provided by nanotechnology for the treatment of IDs on Nature Nanotechnology, and discussed the preclinical and clinical progress in this field. The focus is on HIV, TB and malaria. In addition, the researchers also recognized that nanotechnology is being successfully applied to SARS-CoV-2 mRNA and protein vaccines. Finally, they discussed the challenges of applying these techniques from the laboratory to the clinic.
The availability and correct use of safe and effective drugs for the treatment of infectious diseases by nanotechnology are necessary conditions for the treatment of IDs. Nanotechnology-based methods have become the subject of intensive preclinical evaluation to increase the therapeutic index of ID drugs and simplify their use. Researchers focus on promising preclinical research and discuss the challenges of its clinical translation.
Continuous systemic administration of anti-infectious drugs The administration of many infectious diseases involves long-term treatment, which places a heavy burden on patients and the healthcare system. For example, HIV and tuberculosis, which lead to lower patient compliance, and ultimately lead to treatment failure. Therefore, the development of a system that reduces the frequency of doses and simplifies the dosing schedule has attracted widespread interest.
At present, scientists have been actively seeking injectable nanocarriers that can continuously deliver drugs. Broadly speaking, these systems can be divided into two categories: systems that control drug release through excipients (such as polymers or lipids), and systems that rely on poorly soluble drug crystals to slowly dissolve in the tissue fluid.
Quite a lot of research has focused on polyester-based and liposomal drug delivery systems. The disadvantage of polymer and liposome formulations is that they require a large amount of excipients to control drug release. This increases the volume of the injection and limits the dosage of the drug. An important consideration for sustained-release systems is the possibility of long-term exposure to sub-therapeutic concentrations, which may lead to drug resistance.

Figure | Nanocarriers for continuous local and systemic drug delivery
Local administration As the epithelial surface is a common site for pathogens to enter and reside, regional drug delivery to these surfaces has broad prospects. Compared with systemic administration, local delivery to the site of infection may increase (and minimize off-target) drug exposure. The current research involves: a vaginal local drug delivery system that manages to quickly pass through the mucosa, pulmonary delivery to treat respiratory infections, and topical topical treatment of wound infections.
Targeted delivery to the site of infection has aroused widespread research interest in the design of systems that can deliver drugs to the site of infection in a targeted manner. This is due to the low penetration rate of the drug into the infected tissue (for example, the low penetration rate of antiretroviral drugs to the brain and lymph nodes and the low penetration rate of anti-tuberculosis drugs to cavity lesions), the distribution of the drug into the toxic site and the symbiotic microorganisms Caused by killing. Encapsulation in nanocarriers provides the possibility of drug targeting, but there is still much room for improvement. Compared with uninfected tissues, due to the enhanced permeability of nanocarriers at the infected site, better targeted aggregation occurs. The surface of the nanocarrier can also be functionalized with ligands that bind to infected tissues or microorganisms. The latter strategy is called active targeting or ligand-mediated targeting. Therefore, strategies that do not rely on specific ligands are called passive targeting.

Figure | Nanocarriers for targeted drug delivery
Overcoming resistance to anti-infectives Overcoming resistance is a key and growing obstacle to the treatment of IDs. Drug resistance is caused by the secretion of drug-inactivating enzymes and the transformation of pathogens into a state of reduced metabolic activity, the formation of biofilms, the use of intracellular life cycles, or obligate intracellular pathogens.
Drugs spread poorly on the host cell membrane, and active efflux through drug transporters can reduce the activity against intracellular pathogens. Another complication is that drug molecules and pathogens are unevenly distributed in the host cell, and without co-localization, the efficacy is affected. For example, aminoglycosides accumulate in large amounts in lysosomes and may be ineffective against cytoplasmic pathogens. Therefore, strategies to enhance intracellular accumulation of drugs and target subcellular locations may improve the efficacy of anti-infective drugs.

Figure | Nanocarriers overcome drug resistance
Under advanced preclinical and clinical nanotechnology, researchers will focus on the application of the most advanced nanotechnology in HIV infection, tuberculosis and malaria. Readers will learn that nanotechnology has received the most active research in clinical and large animals for the treatment and prevention of HIV infection. In contrast, for malaria and tuberculosis, peoples pursuit of nanotechnology is less enthusiastic. For these IDs, work is mainly limited to preclinical trials in rodents. The researchers also hope to convey that the most advanced nanotechnology is not complicated, but it must have a strong influence. Therefore, perhaps in order for nanotechnology to benefit patients, it needs to meet certain principles, such as simple design, clinical needs, and economic enthusiasm.
AIDS HIV infection is one of the main IDs leading to high global morbidity and mortality. Long-acting injection of antiretroviral drugs is the most clinically advanced nanotechnology in HIV treatment. Long-acting injectable nanoparticles reduce the frequency of dosing and can improve patient compliance. This is widely regarded as a breakthrough intervention. In addition, oral antiretroviral nanoparticles have been developed for use in pediatrics, aiming to replace tablets (difficult to swallow) and alcohol-containing solutions (containing harmful excipients).

Figure | HIV life cycle
Malaria Malaria caused by a single-celled complex protozoan of the genus Plasmodium is the most prevalent parasitic disease in the world. Although more than 3 billion people are at risk of infection, funding for the global malaria response has been stagnant since 2010, reaching only US$3.1 billion in 2017. As part of the investment, short- and long-term malaria solutions must be considered, such as the use of nanotechnology for treatment and vaccination. Considering that the target product price for the treatment of malaria is US$1 per day, the key consideration for the development of a new method is its cost-effectiveness.

Figure | The life cycle of malaria in two hosts
Tuberculosis Tuberculosis kills more than 3,500 people every day and is the worlds top killer of ID. According to data from the World Health Organization (WHO), 10 million people suffered from tuberculosis in 2017, and the global economic burden reached 12 billion U.S. dollars each year. In addition, Mycobacterium tuberculosis is the most critical pathogen in the global antimicrobial resistance crisis.
WHO guidelines recommend oral medications of four antibiotics per day to treat drug-sensitive tuberculosis for at least 6 months. Due to long-term and frequent dosing and side effects, patients find it difficult to adhere to these treatment regimens and are at risk of developing resistant strains. Therefore, reformulating existing drugs in nanocarriers is an attractive method that has a direct impact on tuberculosis treatment. Several systems based on oral nanotechnology have been designed using existing tuberculosis drugs and tested in animal models. In addition, nanocarriers for inhalable tuberculosis drugs have always been the focus of research. Currently, there is no effective vaccine to prevent tuberculosis infection in an all-round way. Researchers are trying to improve the existing BCG vaccine and derive new vaccines to prevent TB.
Figure | Pathogenesis of tuberculosis infection
Summary and Prospects The above highlights many promising therapeutic strategies for developing nanomedicine to treat and prevent ID. However, in order to have the greatest impact, nanotechnology solutions will need to overcome some economic cost, manufacturing, and regulatory challenges. 1) First, reducing costs will be the main obstacle to the development of new drugs and nanotechnology-based systems. The currently approved nanosystem (an amphotericin B liposome formulation) is not cost-effective in developing countries. 2) Second, the synthesis and storage conditions of some nanoparticles may not be conducive to the conditions of resource-poor countries. 3) Third, in the clinical trial phase, regional and national differences within and between regulatory agencies, especially when conducting multi-center international trials, will be a challenge. 4) Finally, the patient acceptability of these nanosystems needs to be addressed.
In the new crown epidemic, nanotechnology has had a significant impact, which provides broad prospects. Similarly, similar feats of nanotechnology are possible in the treatment of other infectious diseases.
It is worth noting that the research group recently published another research paper on Nature Nanotechnology. They reported on the integration of machine learning and high-throughput experiments to quickly and large-scale identification of such nano-agents. The researchers identified 100 self-assembled drug nanoparticles from 2.1 million pairs, each of which included one of 788 drug candidates and one of 2,686 approved excipients.
Introduction:
Giovanni Traverso is an assistant professor in the Department of Mechanical Engineering at the Massachusetts Institute of Technology (MIT) and a gastroenterologist in the Department of Gastroenterology, Brigham and Womens Hospital, Harvard Medical School. Dr. Traversos previous work focused on the development of new molecular detection methods for the early detection of colon cancer. During his postdoctoral research, he transitioned to the fields of chemistry and biomedical engineering in the laboratory of Professor Robert Langer of the Massachusetts Institute of Technology, where he developed a series of novel technologies for drug delivery and physiology through the gastrointestinal tract Sensing.
His current research plan focuses on developing next-generation drug delivery systems to deliver therapeutic drugs safely and effectively, and developing novel ingestible electronic devices to sense a wide range of physiological and pathophysiological parameters. In addition, Dr. Traverso continues to work on the development of new diagnostic tests that can detect cancer early.
References: 1. Kirtane, AR, Verma, M., Karandikar, P. et al. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. (2021).https://doi.org/10.1038/s41565-021- 00866-82. Reker, D., Rybakova, Y., Kirtane, AR et al. Computationally guided high-throughput design of self-assembling drug nanoparticles. Nat. Nanotechnol. (2021).https://doi.org/10.1038 /s41565-021-00870-y
- Previous: Guo Baolin, Xian Jiaot
- Next: IF 26.8! A dual-action


 Academic Frontier
Academic Frontier
