Aenm: graphene iridium oxide quantum dot catalyst for efficient acid total hydrolysis
QQ Academic Group: 1092348845
Detailed
Energy is an important material basis for the survival and development of human society. The development of catalysts with high activity, high stability and high selectivity is the key to break through the current research bottleneck and realize the efficient utilization of green and sustainable energy such as hydrogen energy. Graphene (GDY) is a new carbon material with independent intellectual property rights in China. Chinese scientists lead the development of this field. Due to its unique chemical and electronic structure, graphene has abundant natural active sites, large specific surface area, excellent hole transport ability and other excellent properties, showing natural advantages in the fields of photocatalysis / electrocatalysis, solar cells, energy storage and conversion.
Recently, the Li Yuliang research group of the Institute of chemistry of the Chinese Academy of Sciences achieved uniform and controllable growth and highly dispersed distribution of iridium oxide quantum dots through a new strategy of graphene induced metal atom anchoring in situ nucleation growth of quantum dots , and obtained three-dimensional flexible graphene iridium oxide quantum dot catalyst (iroxqd / GDY). The results show that graphene can effectively regulate the coordination environment and valence state of metal atoms in QDs, improve their charge transfer behavior and maximize the number of reactive sites. Therefore, the obtained iroxqd / GDY catalyst exhibited excellent performance and stability in acid oxygen evolution and acid total hydrolysis, for example, in 0.5 m H2SO4 electrolyte, Iroxqd / GDY as anode and cathode materials can achieve 10 mA / cm2 current density at a low voltage of 1.49 V (vs. rhe) and almost no degradation after 30000 CV cycles. The performance of the catalyst is better than that of the reported catalyst. The research work provides a new idea for the design of a new type of high performance acid electrolytic cell.
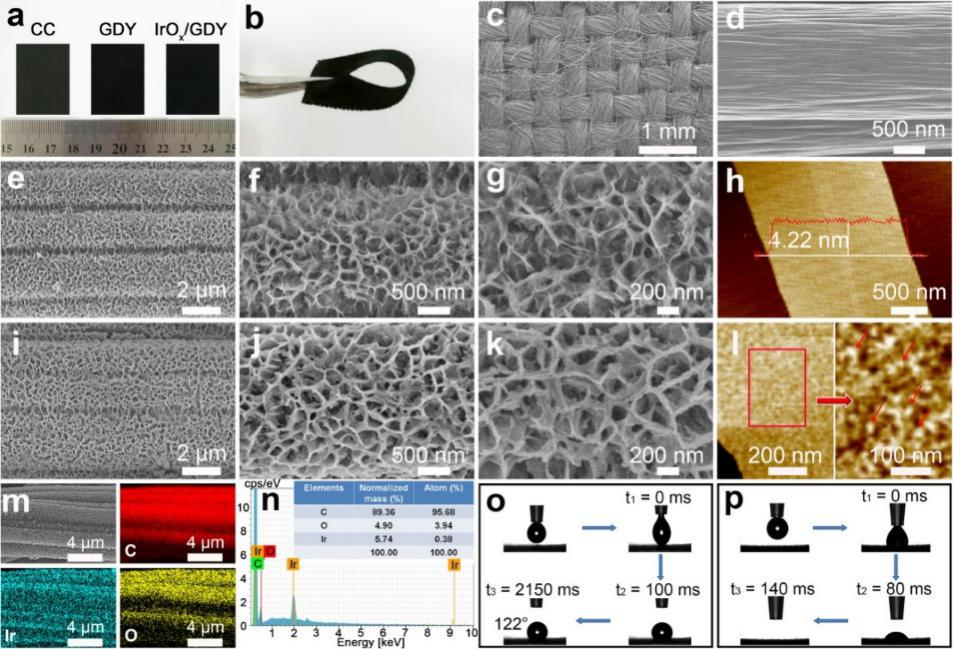
Fig. 1 (a) optical photos of the experimental sample and (b) iroxqd / GDY electrode( c. D) SEM images of carbon cloth, (E-G) graphene, and (i-k) iroxqd / GDY( h) AFM images of graphene and (L) iroxqd / GDY( m) The element distribution of iroxqd / GDY samples was analyzed( n) EDS spectrum of iroxqd / GDY (attached figure: contents of C, O and IR in the sample)( o) Contact angle test of carbon cloth and (P) iroxqd / GDY.
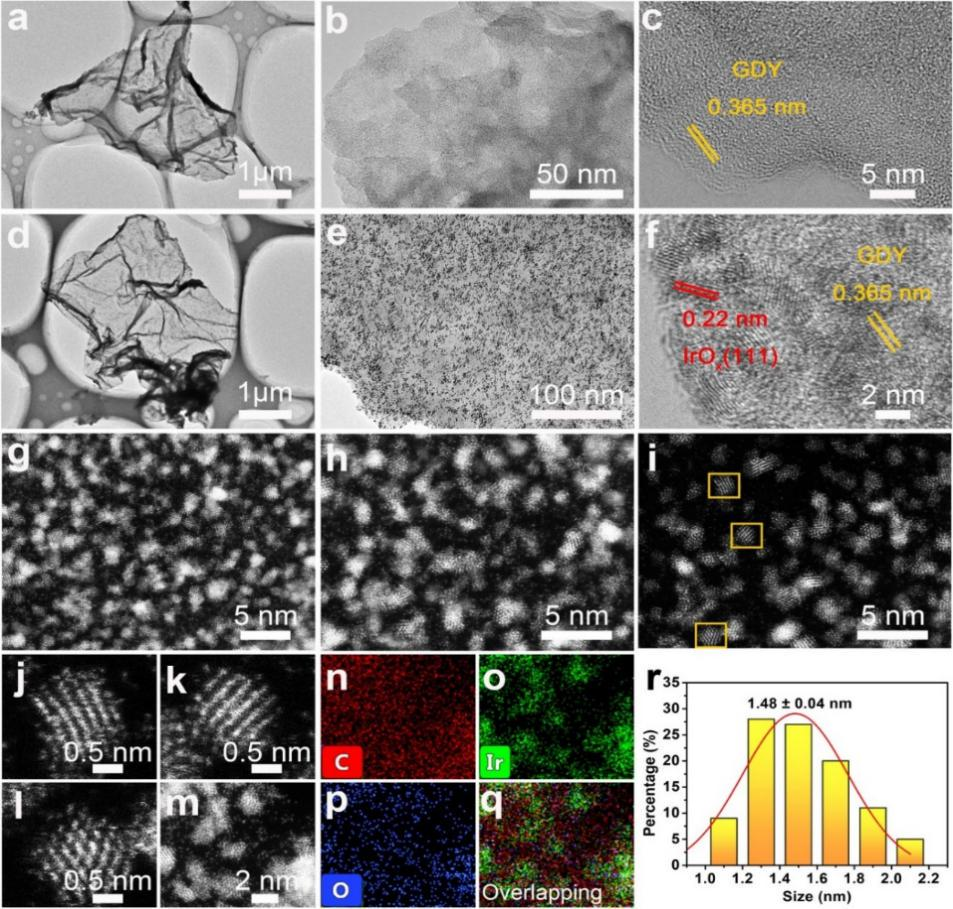
Fig. 2. (a) tem and (B, c) HRTEM images of graphene; (d) tem, (E, f) HRTEM, and (G-L) HAADF-STEM images of iroxqd / GDY; The (m) stem and (n-p) element mapping image of iroxqd / GDY and its (q) overlapping image( r) The particle size distribution of iroxqd supported on graphene is 1.48 ± 0.04 nm.
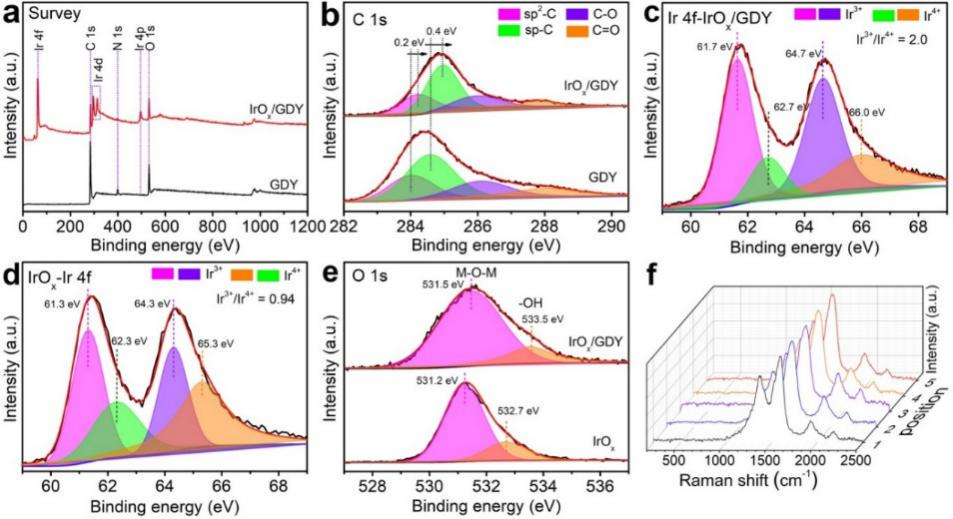
Fig. 3 (a) XPS full spectrum data and (b) C 1s XPS fine spectrum of graphene and iroxqd / GDY( c) The IR 4f XPS fine spectra of iroxqd / GDY and (d) iroxqds were obtained( e) The o 1s XPS fine spectra of iroxqd / GDY and iroxqds were obtained( f) Raman spectra of graphene. The results show that graphene is beneficial to obtain IR3 + / IR4 + mixed valence with iroxqd / GDY; Moreover, the structure of graphene is complete.
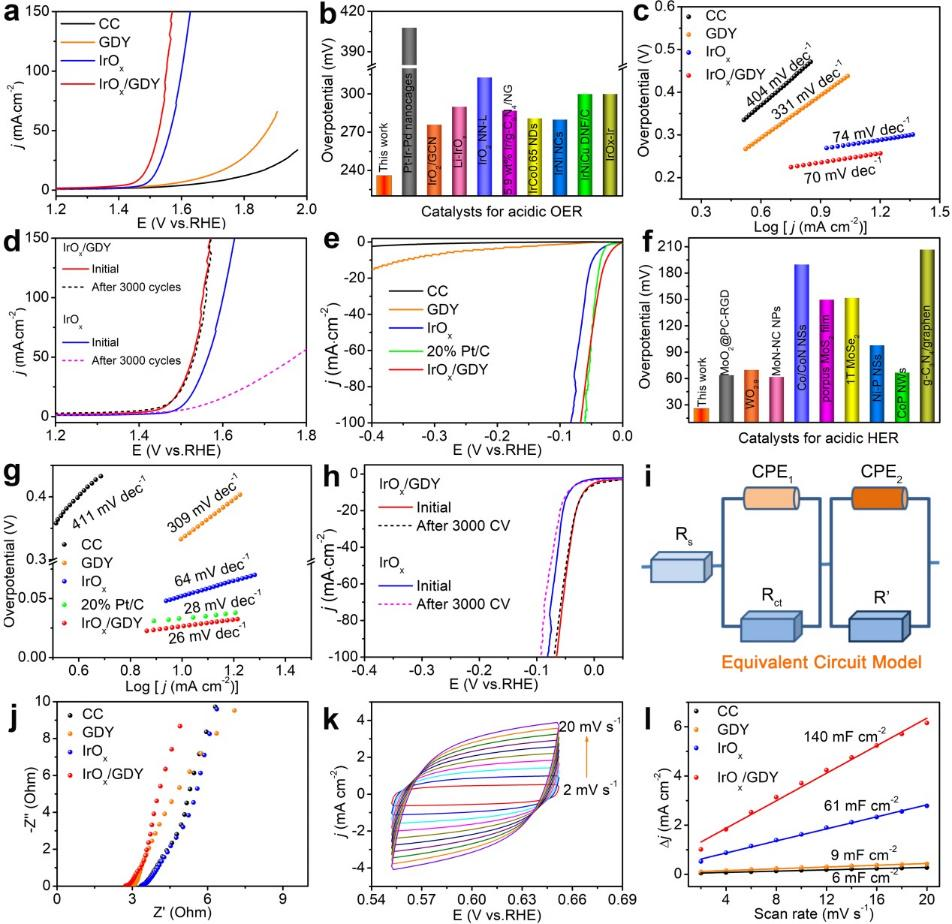
Fig. 4 (a) linear sweep polarization curve of acid oer of catalyst and its performance comparison with reported catalyst (b)( c) Tafel slope of catalyst( d) CV cycle stability of iroxqd / GDY and iroxqds in Aoer( e) LSV curve of her and its performance comparison with the reported catalyst (f)( g) Tafel slope of catalyst( h) The CV cycle stability of iroxqd / GDY and iroxqds in acidic her was studied( i) R (QR) (QR) equivalent circuit model( j) Nyquist impedance diagram( k) The cyclic voltammetry curve of iroxqd / GDY was obtained( l) The relationship between capacitive current density and scanning rate.
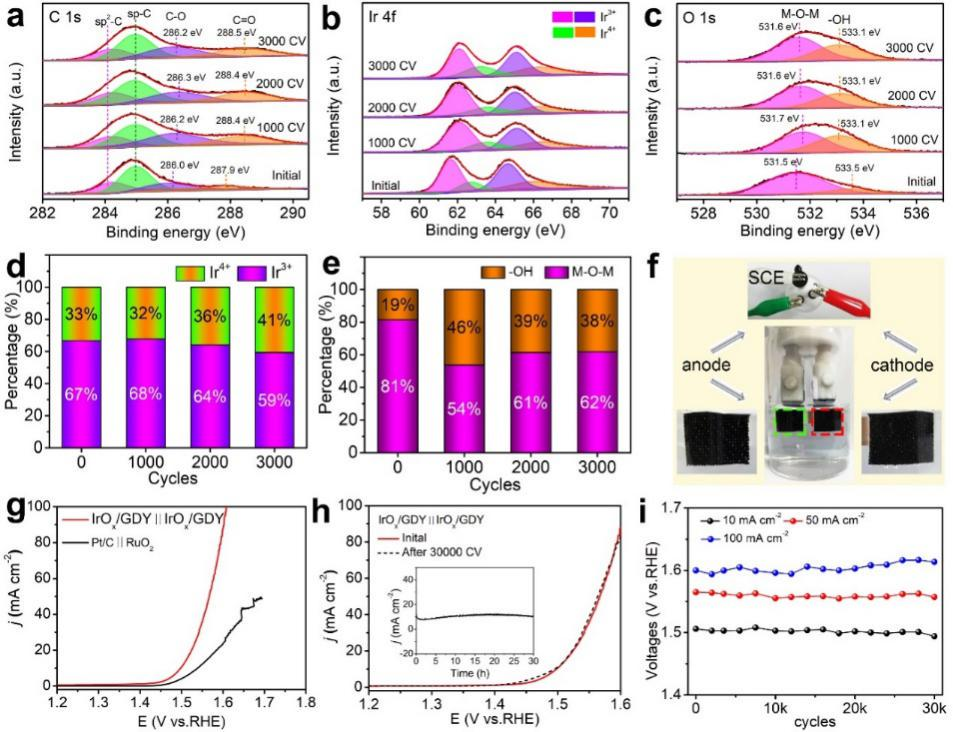
Fig. 5 XPS fine spectra of (a) C 1s, (b) IR 4f, and (c) O 1s after different cycles in CV test of acid oxygen evolution reaction; The percentages of (d) IR3 + / IR4 + and (E) - OH / M-O-M before and after CV were statistically analyzed( f) Complete hydrolysis unit diagram( g) Iroxqd / GDY ∥ iroxqd / GDY and Pt / C ∥ RuO2 were compared( h) Stability test of acid electrolytic cell( i) The battery voltage changes during CV test.
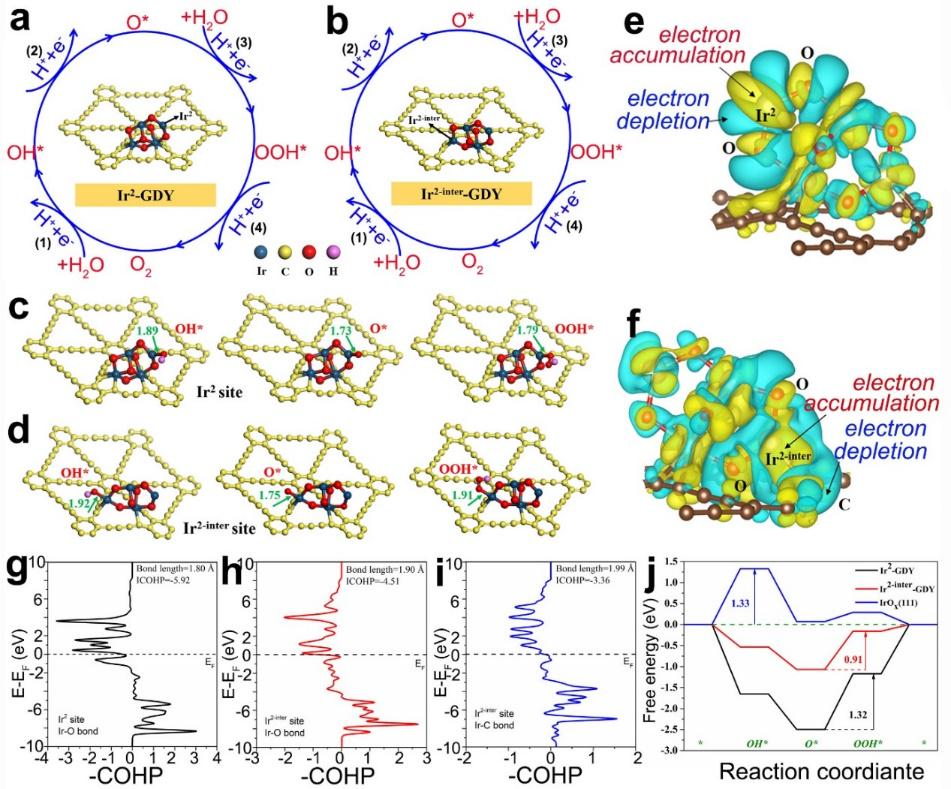
Fig. 6 four successive electronic steps of (a) non interface and (b) interface iridium sites; The adsorption of active intermediates (OH *, O *, ooh *) on (c) IR2 site and (d) IR2 inter site was investigated( e) The differential charge density map of IR2 site and (f) IR2 inter site; The crystal orbital Hamiltonian population diagram (Cohp) of (g) IR2 site (h, I)) IR2 inter site in iroxqds / GDY( j) When u0rhe = 1.23 V, the free energy diagram of Aoer at IR site.
This study proved the natural advantages of graphene in the controllable synthesis of metal oxide quantum dot catalysts and in the process of improving the performance of the catalysts, and provided a new idea for the controllable preparation of new efficient quantum dot catalysts.
Li Yuliang, academician of Institute of chemistry, Chinese Academy of Sciences, Xue Yurui, Professor of Shandong University and he Feng, associate researcher of Institute of chemistry, Chinese Academy of sciences are the co corresponding authors.
This information is from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately
- Previous: Infomat cover article
- Next: IF 12.9! Dual-nanopart


 Academic Frontier
Academic Frontier
