ASC NANO: High-performance zinc-ion battery based on V2CTX MXene in-situ growth of MnO2 nanosheets
QQ Academic Group: 1092348845
Detailed

one. Article overview
The manganese-based zinc ion battery has the advantages of simple preparation, low cost and high safety, and is a large-scale rechargeable battery with broad application prospects. However, the low-rate performance and poor cycle performance of manganese-based cathodes hinder the commercial application of manganese-based cathodes. In this paper, metal ion intercalation in-situ growth technology was used to form MnO2 nanosheets uniformly on the surface of V2CTX MXene, and a manganese-vanadium hybrid cathode K -V2C@MnO2 was designed and synthesized. K-V2C@MnO2 has a hybrid structure with high conductivity and abundant active sites, as well as the synergistic Mn2+ electrodeposition reaction and the inhibition of MnO2 structural damage. It shows excellent electrochemical performance for ZIBs in water. Specifically, it exhibits a high specific capacity of 408.1 mAh g-1 at 0.3 A g-1, and maintains 119.2 mAh g-1 at a high current density of 10 A g-1 for 10,000 long-term cycles. The specific capacity. It is superior to almost all ZIBs reported in manganese-based cathode aqueous solutions. Its excellent electrochemical performance shows that the manganese-based cathode material designed in this article is a reasonable method and can be used for high-performance ZIB cathodes.
Two, graphic guide
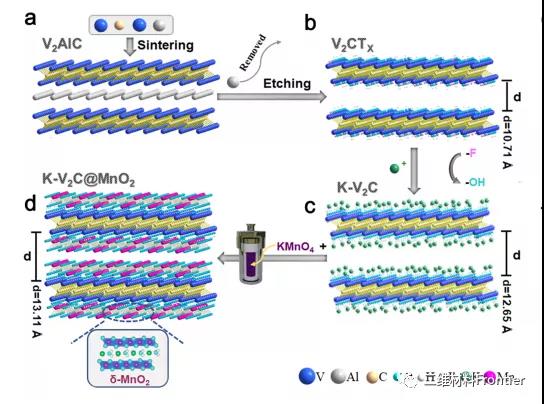
Figure 1 shows the synthesis diagram of .K -V2C@MnO2. (a) The structure of the V2AlC MAX phase obtained by sintering V, Al, and c. (b) V2CTX MXene obtained by hf etching. (c) The interaction between alkalized V2CTX and K+ ions. (d) MnO2 modified pre-alkalized V2CTX through the template effect and in-situ growth process.
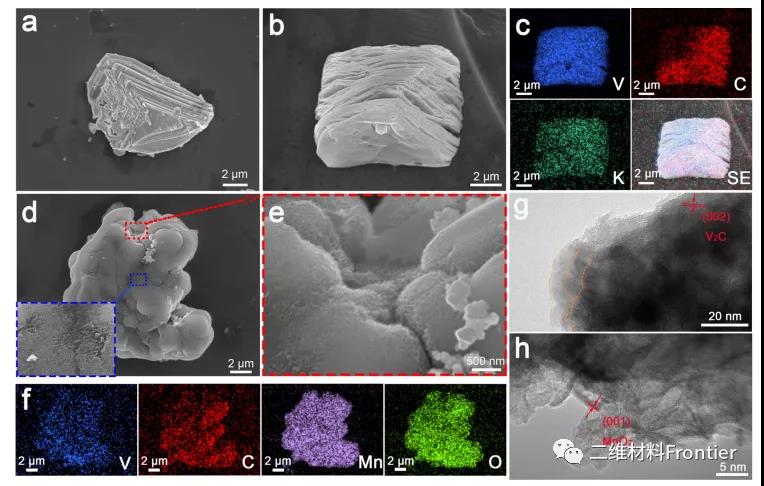
Figure 2. SEM images of (a) V2AlC MAX phase and (b) kv2c phase. (c) Element mapping corresponding to V, c, K (d, e) K V2C@MnO2 SEM image (f) Element mapping corresponding to V, C, Mn, O. (g) HRTEM image of K−V2C@MnO2. (h) HRTEM image of K−V2C@MnO2 with higher magnification.
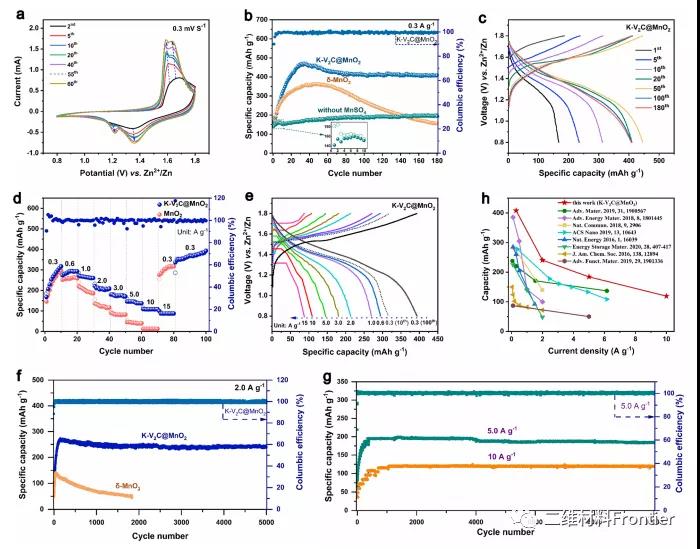
Figure 3. (a) After different cycles, the CV curve of the K−V2C@MnO2 electrode at a scan rate of 0.3mV−1. (b) Cycle performance of K−V2C@manganese dioxide, K−V2C@K−V2C@MnO2 and δ-MnO2 electrodes without MnSO4 under 0.3Ag−1. (c) Constant current charge and discharge curve of K−V2C@MnO2 at 0.3Ag−1. (d) Rate performance of δ-MnO2 and K−V2C@MnO2 electrodes. (e) Constant current charge and discharge curves of K−V2C@MnO2 electrode at different current densities. (f) The long-term cycle performance of δ-MnO2 and K−V2C@MnO2 electrodes under 2.0Ag−1 conditions. (g) The long-term cycling performance of K−V2C@MnO2 at a fairly high current density. (h) Compared with the reported Mn-based cathode, the specific capacity of the K−V2C@MnO2 electrode is stable at different current densities.

Figure 4. (a) CV curve of K−V2C@MnO2 electrode at different scan rates. (b) The logi and logv plots at a specific peak current. (c) The contribution ratio of capacitance capacity and diffusion control capacity in kV2C@MnO2 electrode. (d) GITT curves and corresponding ion diffusion coefficients of kV2C@MnO2 and δ-MnO2 under charge and discharge conditions. (e) Nyquist diagrams of K-V2C@MnO2 and δ-MnO2 cathodes at the initial open circuit potential (the inset is the equivalent circuit of the fitted electrical impedance spectrum). (f) Band edge position of δ-MnO2 and V2C. (g) DOS of δ-MnO2, V2C and MnO2-V2C. (h) Calculate the absorption energy of Zn2+ on the surface of MnO2-V2C and δ-MnO2.
Third, the full text summary
In summary, the manganese-based cathode material of MnO2 nanosheets coated with layered V2CTX MXene is beneficial to improve the specific capacity, rate performance and cycle life of zib. Using the strategy of K+ ion intercalation and in-situ growth, an advanced K-V2C@MnO2 hybrid was successfully synthesized, which is an advanced manganese-vanadium composite zinc ion storage cathode. Physical characterization and electrochemical research k-V2C@MnO2 electrode has considerable structural stability and high reversibility. In addition, combined with electrical analysis, simulation calculation, synchronous XANES and EXAFS technology, the Zn2+ insertion/extraction mechanism and phase transition of the K V2C@MnO2 electrode are explained. Compared with most existing ZIBs cathode materials, K−V2C@MnO2 has the advantages of a conductive substrate and high surface activity, as well as excellent rate performance, high capacity and cycle stability. Specifically, K−V2C@MnO2 and Mn2+ electrodeposition enhance the synergistic reaction and suppress structural damage by manganese dioxide, reaching a magical capacity of 408.1mAhg−1 at 0.3Ag−1, which is the best Zn2+ for manganese cathodes reported previously. capacity. In addition, even at a fairly high rate of 10Ag−1, K−V2C@MnO2 still maintains an achievable capacity of 119.2mAhg−1 after 10,000 cycles. The results show that K−V2C@MnO2 is a promising high-performance zib cathode material. In addition, this work not only provides an idea for further understanding of the zinc ion insertion process, but also provides a method for the development of low-cost, high reversibility, high stability and safety water batteries.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
- Previous: Jiang Hulin AM, China
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier