The team of Professor Feng Jinkui of Shandong University ACS NANO: Design a three-dimensional sulfur-doped MXene/ZnS heterostructure as a multifunctional protective layer for zinc anode
QQ Academic Group: 1092348845
Detailed


Article information
Designing a three-dimensional sulfur-doped MXene/ZnS heterostructure as a multifunctional protective layer for zinc anodes First author: An Yongling Corresponding author: Feng Jinkui Unit: Shandong University
Research Background
Because zinc anode has many advantages such as high theoretical reversible capacity, low oxidation-reduction potential, low cost, abundant resources, and environmental friendliness, water-based zinc metal batteries are widely regarded as one of the promising candidate batteries. However, uneven zinc deposition can cause severe dendrite growth and huge volume expansion, resulting in low Coulomb efficiency and short circuits. In order to solve the problem, researchers have proposed many methods to extend the service life of zinc anodes, such as introducing an interfacial electric field, adjusting the coordination environment, and inducing uniform zinc deposition. However, these methods are more complicated and difficult to implement on a large scale. Therefore, there is an urgent need to propose a simple and effective method to inhibit the growth of zinc dendrites.
Article Introduction
Based on this, the team of Professor Feng Jinkui of Shandong University published a research article titled "Rational Design of Sulfur-Doped ThreeDimensional Ti3C2Tx MXene/ZnS Heterostructure as Multifunctional Protective Layer for Dendrite-Free Zinc-Ion Batteries" in the internationally renowned journal ACS Nano. By designing the heterostructure of electronically conductive three-dimensional sulfur-doped MXene and ion-conductive ZnS, it tries to solve the problems of zinc metal batteries. Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. Based on this, a zinc negative electrode with a long cycle life was obtained.
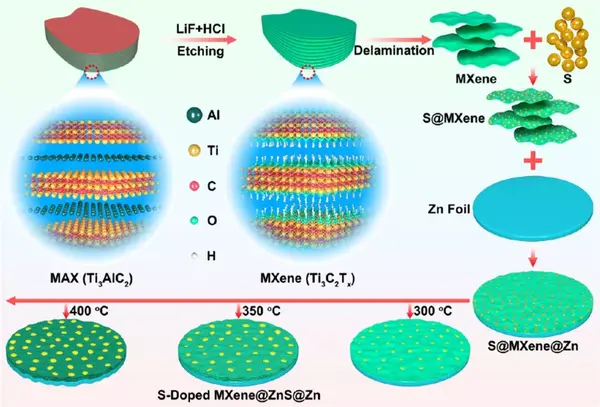
Figure 1 Schematic diagram of synthesis of S/MX@ZnS@Zn at different temperatures
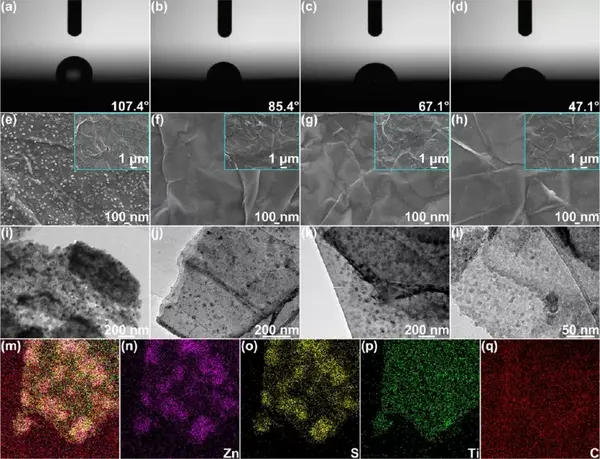
Figure 2 Synthesis and characterization of the product S/MX@ZnS@Zn. (Ad) (a) Commercial zinc foil, (b) S/MX@ZnS@Zn-300, (c) S/MX@ZnS@Zn-350 and (d) S/ in 2M zinc sulfate electrolyte The contact angle of MX@ZnS@Zn-400. (E) SEM images of S@MX@Zn, (f)S/MX@ZnS@Zn-300, (g)S/MX@ZnS@Zn-350 and (h)S/MX@ZnS@Zn-400 . TEM images of (i) S@MX@Zn, (j)S/MX@ZnS@Zn-300, (k)S/MX@ZnS@Zn-350 and (l)S/MX@ZnS@Zn-400 . (M-q) STEM-EDS element distribution diagram of S/MX@ZnS@Zn-350.
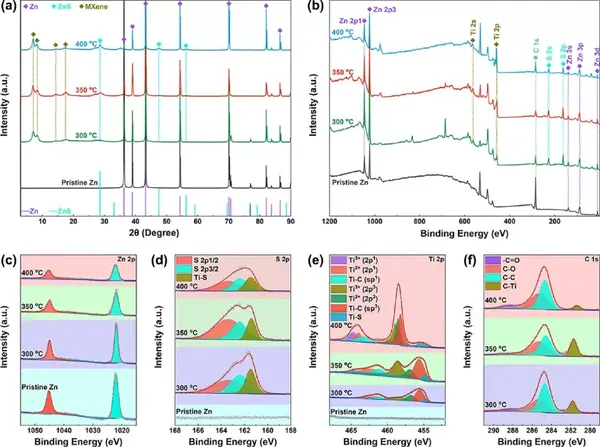
Figure 3 Structural characterization of the product S/MX@ZnS@Zn. (A) XRD diagram, (b) XPS diagram, (c) Zn 2p diagram, (d) S 2p diagram, (e) Ti 2p diagram and (f) C 1s diagram.
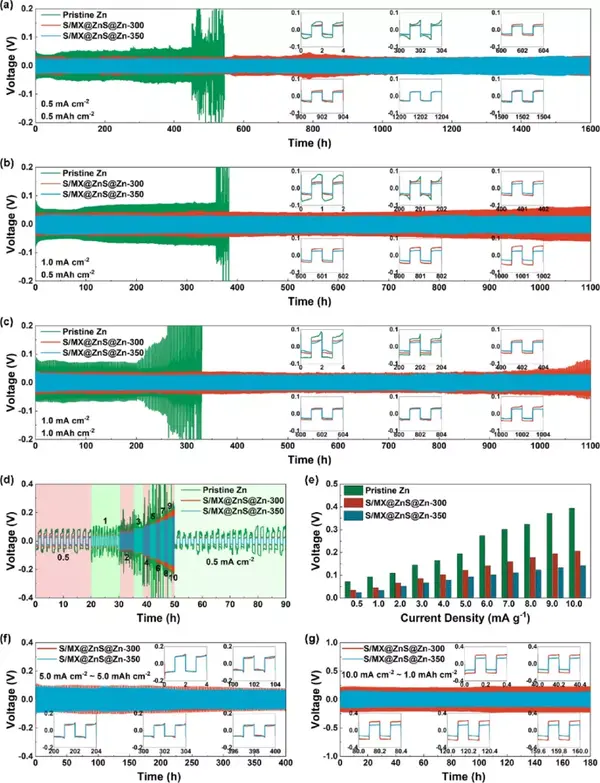
Figure 4 The electrochemical performance of commercial zinc foil and S/MX@ZnS@Zn anode. (A-c) Cycle performance under different current densities. (D) Rate performance and (e) polarization voltage under different current densities. (F-g) Cycle performance under high current density.
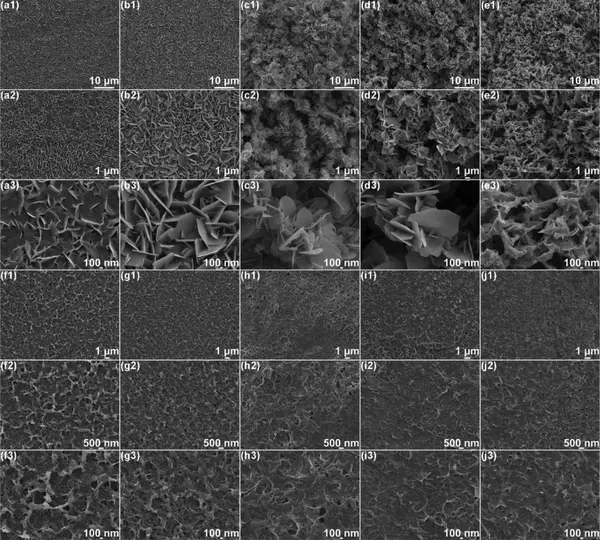
Figure 5 (a-e) SEM images of the structure evolution of commercial zinc foil and (f-j) S/MX@ZnS@Zn anode under different zinc deposits. (A1−a3, f1−f3) 1 h, (b1−b3, g1−g3) 2 h, (c1−c3, h1−h3) 5 h, (d1−d3, i1−i3) 10 h, (e1 −e3, j1−j3) 20 h
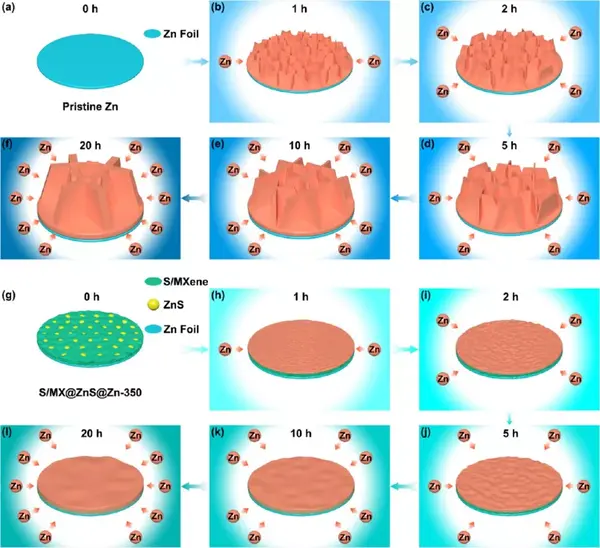
Figure 6 (a-f) Schematic diagram of structural evolution of commercial zinc foil and (g-l) S/MX@ZnS@Zn anode under different zinc deposits. (A, g) 0 h, (b, h) 1 h, (c, i) 2 h, (d, j) 5 h, (e, k) 10 h, (f, l) 20 h
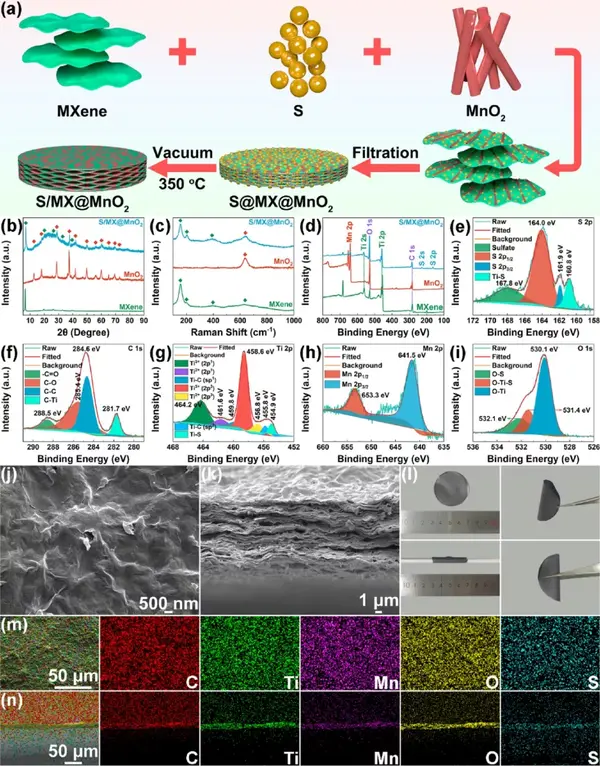
Figure 7 Synthesis and characterization of S/MXene@MnO2 cathode. (A) Synthesis diagram of MXene, MnO2, S/MXene@MnO2. (B) Raman diagram, (c) XPS diagram. S/MXene@MnO2 high magnification (e-i) XPS image, (j-k) SEM image, (l) optical photo image, (m-n) element distribution diagram.
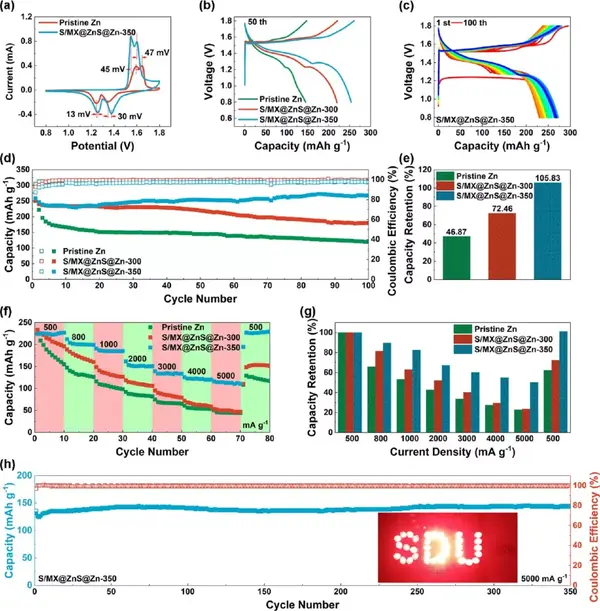
Figure 8 The electrochemical performance of the full battery (the positive electrode is S/MXene@MnO2, and the negative electrode is commercial zinc foil or S/MX@ZnS@Zn). (A) Cyclic voltammetry curve, (b) charge and discharge curve at the 50th week. (C) Charge and discharge curve. (D) Cycle performance and (e) the corresponding capacity retention rate. (F) Rate performance and (g) corresponding capacity retention rate. (H) Long cycle performance.
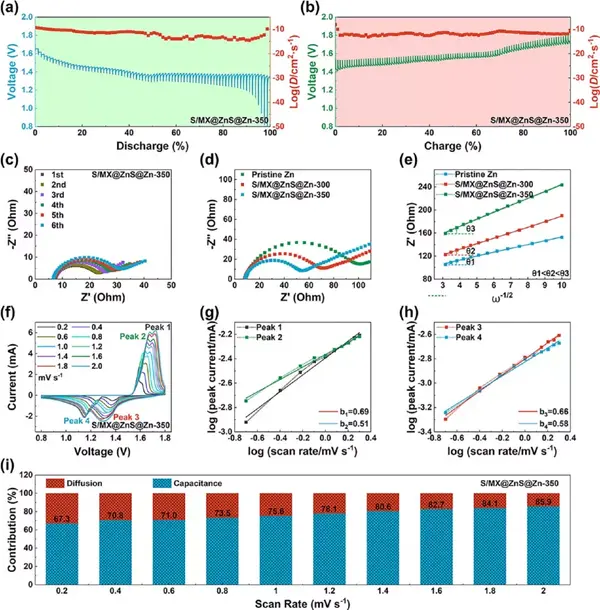
Figure 9 The electrochemical mechanism analysis of the full battery. (A-b) GITT curve and diffusion coefficient. (C) The EIS curve for the first five weeks. (D) EIS curve and (e) the relationship between Z′ and ω−1/2. (F) Cyclic voltammetry curves at different scanning speeds. (G-h) The relationship between peak current and scanning speed. (I) The ratio of pseudocapacitance and diffusion capacitance at different scanning speeds.
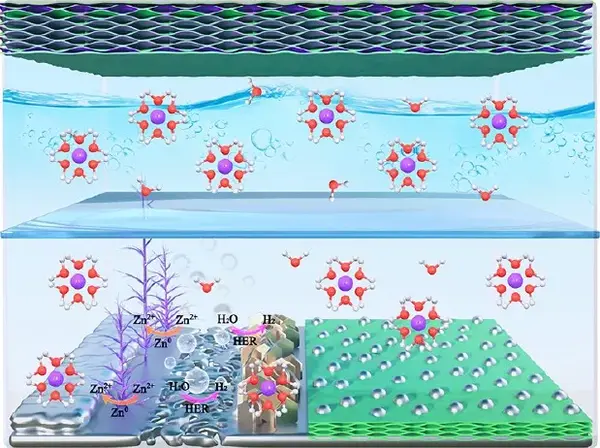
Figure 10 Schematic diagram of water-based rechargeable zinc battery (positive electrode is S/MXene@MnO2, negative electrode is commercial zinc foil or S/MX@ZnS@Zn)
Highlights of this article
1 Construct a multifunctional protective layer for electronic and ion conduction. Through the vacuum distillation method, a three-dimensional sulfur-doped MXene/ZnS heterostructure is obtained in one step. The sulfur doping and porous structure are obtained simultaneously with the synthesis of ZnS. By changing the reaction conditions, the microstructure and electrochemical properties of the product can be adjusted. 2 Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. 3 Build a high-rate water-based zinc-ion battery. Using the synthesized self-supporting S/MXene@MnO2 as the positive electrode and S/MX@ZnS@Zn as the negative electrode, a high-rate full battery was obtained.
in conclusion
A multifunctional protective layer with ion and electronic conductivity is constructed and used in water-based zinc metal batteries. (1) Sulfur doping and zinc sulfide production are carried out simultaneously during the preparation process. (2) Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. (3) ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. (4) In water-based zinc ion batteries, the protective layer can exist stably, and the reversibility of the zinc anode can be improved by suppressing side reactions and uniform zinc deposition. Based on the above design, the modified zinc anode can be cycled stably for more than 1600 h. In addition, a self-supporting S/MXene@MnO2 is used as the positive electrode to assemble a high-rate zinc ion battery.
Article link
Yongling An, Yuan Tian, Chengkai Liu, Shenglin Xiong, Jinkui Feng*, and Yitai Qian, Rational Design of Sulfur-Doped ThreeDimensional Ti3C2Tx MXene/ZnS Heterostructure as Multifunctional Protective Layer for Dendrite-Free Zinc-Ion Batterieshttps://doi.org /10.1021/acsnano.1c05934
Corresponding author introduction
Highlights of this article
1 Construct a multifunctional protective layer for electronic and ion conduction. Through the vacuum distillation method, a three-dimensional sulfur-doped MXene/ZnS heterostructure is obtained in one step. The sulfur doping and porous structure are obtained simultaneously with the synthesis of ZnS. By changing the reaction conditions, the microstructure and electrochemical properties of the product can be adjusted. 2 Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. 3 Build a high-rate water-based zinc-ion battery. Using the synthesized self-supporting S/MXene@MnO2 as the positive electrode and S/MX@ZnS@Zn as the negative electrode, a high-rate full battery was obtained.
in conclusion
A multifunctional protective layer with ion and electronic conductivity is constructed and used in water-based zinc metal batteries. (1) Sulfur doping and zinc sulfide production are carried out simultaneously during the preparation process. (2) Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. (3) ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. (4) In water-based zinc ion batteries, the protective layer can exist stably, and the reversibility of the zinc anode can be improved by suppressing side reactions and uniform zinc deposition. Based on the above design, the modified zinc anode can be cycled stably for more than 1600 h. In addition, a self-supporting S/MXene@MnO2 is used as the positive electrode to assemble a high-rate zinc ion battery.
Article link
Yongling An, Yuan Tian, Chengkai Liu, Shenglin Xiong, Jinkui Feng*, and Yitai Qian, Rational Design of Sulfur-Doped ThreeDimensional Ti3C2Tx MXene/ZnS Heterostructure as Multifunctional Protective Layer for Dendrite-Free Zinc-Ion Batterieshttps://doi.org /10.1021/acsnano.1c05934
Corresponding author introduction

Professor Feng Jinkui, doctoral supervisor, Shandong Taishan Scholars Young Expert, winner of Shandong Outstanding Youth Fund, deputy director of the Institute of Materials Physical Chemistry, Shandong University.Bachelor, Master and Ph.D. graduated from the School of Chemistry and Molecular Science of Wuhan University from 1999 to 2008. From 2008 to 2012, he was engaged in post-doctoral research at the National University of Singapore and Pennsylvania State University. Mainly research new secondary battery materials, and have achieved a series of innovative results in high-energy density aqueous and non-aqueous secondary batteries. A total of more than 160 SCI papers have been published, and more than 80 SCI papers have been published in Adv. Func. Mater, Energy Storage Mater, ACS Nano, Energy Environ. Sci., Nano Energy and other journals as the 1st corresponding author in the past five years. Three of these papers were selected as the cover of Adv. Func. Mater. Chem. Mater and J. Mater. Chem. A respectively. 5 review articles and 1 chapter in English book. He has cited more than 6,000 times, authorized more than 26 patents, presided over 8 projects above the provincial and ministerial level, and 3 industrialization projects. Served as an editorial board member of Scientific Reports, a sub-Journal of Nature. Applied Science Functional materials letter guest editor, member of the New Chemical Materials Committee of the Chinese Society of Chemical Technology, member of the Battery Professional Committee of the Chinese Electrotechnical Society, member of the American Chemical Society, the Royal Society of Britain, and the International Electrochemical Society. The leader of the first evaluation group of new and old kinetic energy conversion new energy in Shandong Province, the evaluation experts of the National Natural Science Foundation of China, and the key research and development plan of Shandong Province.
First author introduction
First author introduction

An Yongling, a 2019-level doctoral student of Shandong University.The research direction is the controllable preparation, performance and mechanism of high-safety and high-energy density electrode materials. The first author has published more than 20 SCI papers in the top international journals Nano Today, Advanced Functional Materials, Nano Energy, Energy Storage Materials, and ACS Nano. He has cited more than 900 times, including 3 ESI papers. More than 50 patents have been applied for and 21 have been authorized (yonglingan@126.com). https://doi.org/10.1021/acsnano.1c02928
https://doi.org/10.1002/adfm.202101886https://doi.org/10.1016/j.nantod.2021.101094https://doi.org/10.1021/acsnano.0c08336https://doi.org/10.1016/j .ensm.2020.07.006https://doi.org/10.1002/adfm.201908721https://doi.org/10.1021/acsnano.9b06653https://doi.org/10.1021/acsnano.8b08740https://doi.org/10.1021 /acsnano.8b02219https://doi.org/10.1016/j.nanoen.2018.03.036
https://doi.org/10.1002/adfm.202101886https://doi.org/10.1016/j.nantod.2021.101094https://doi.org/10.1021/acsnano.0c08336https://doi.org/10.1016/j .ensm.2020.07.006https://doi.org/10.1002/adfm.201908721https://doi.org/10.1021/acsnano.9b06653https://doi.org/10.1021/acsnano.8b08740https://doi.org/10.1021 /acsnano.8b02219https://doi.org/10.1016/j.nanoen.2018.03.036




Scan the QR code|Follow us
17319402180 WeChat same number
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
17319402180 WeChat same number
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
- Previous: Open Ceramics: Process
- Next: IF: 15.7! Biomimetic D


 Academic Frontier
Academic Frontier
