NATURE COMMUNICATIONS: Fast one-pot synthesis of lithium-ion storage performance of two-dimensional titanium carbide
QQ Academic Group: 1092348845
Detailed


one. Article overview
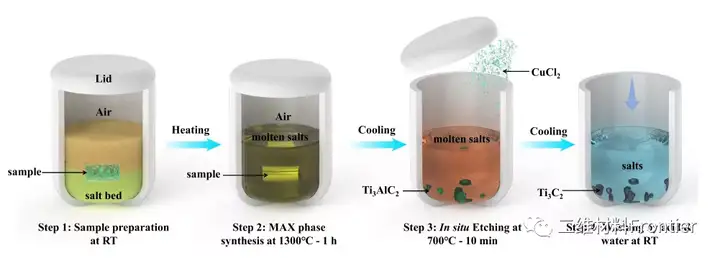
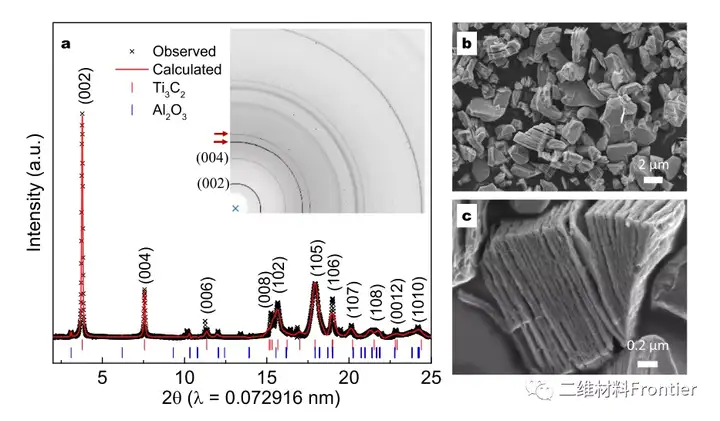
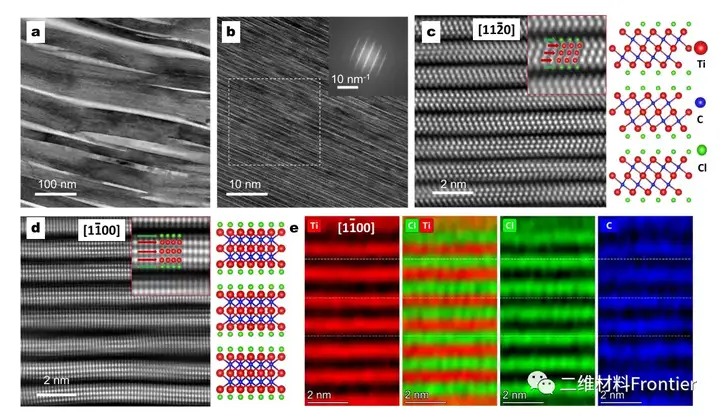
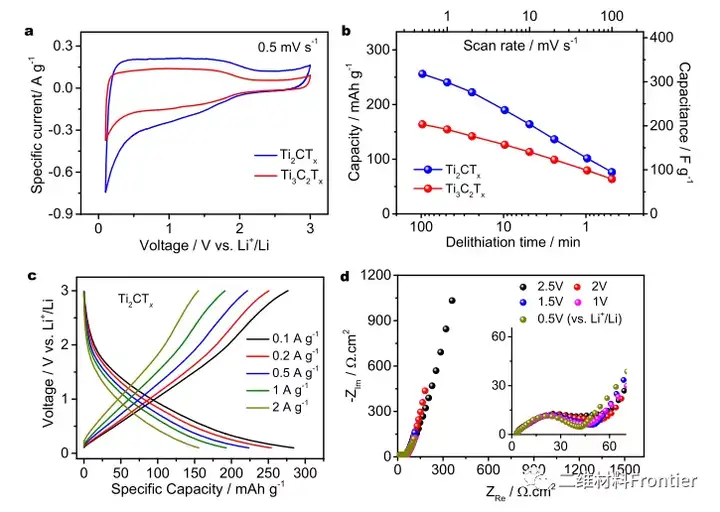
Structural two-dimensional transition metal carbides and/or nitrides (MXenes) have attracted the attention of the material science research community due to their unique physical and chemical properties. However, a simple and cost-effective synthesis of MXene has not yet been reported. Here, using elemental precursors, we report a method of forming aluminum carbide from titanium carbide and subsequently synthesizing MXene by in-situ etching in a molten salt tank. Molten salt is used as the reaction medium to prevent the oxidation of the reactants during the high-temperature synthesis process, so that MXene can be synthesized in an air environment that does not use inert gas protection. The Ti3C2Tx terminal Cl and Ti2CTx MXene are prepared by a one-pot synthesis method, where the 700°C in-situ etching step only takes about 10 minutes. In addition, when used as an active material for non-water lithium ion storage in a half-cell structure, the obtained Ti2CTx MXene exhibits lithiation capacities of approximately 280mAhg-1 and 160mAhg-1 at specific currents of 0.1Ag-1 and 2Ag-1, respectively value.
3. Full text summary
This article proposes a simple one-pot synthesis of element precursors to prepare MXene. By eliminating the need for inert gas protection, the synthesis operation greatly simplifies the synthesis operation. Compared with the traditional MXene synthesis method prepared separately, the single-pot synthesis method shortens the entire synthesis time. The cl end Ti3C2Tx and T2CTx MXenes use a fast etching step at 700°C, only 10 minutes, and the overall synthesis time is less than 8h. Lithium ion storage studies have shown that the one-pot synthesis of MXenes and the previously reported MXenes obtained by Lewis molten acid etching have similar electrochemical characteristics. The obtained Ti2CTx MXene provides lithiation capacity values of approximately 280mAhg-1 and 160mAhg-1 at specific currents of 0.1Ag-1 and 2Ag-1, respectively. We believe that the single-pot synthesis method paves the way for the rapid reduction of production costs of MXene materials, revealing the broad potential of MXene materials in energy storage applications.
Article link:
https://doi.org/10.1038/s41467-021-25306-y



- Previous: ACS Appl. Mater. Inter
- Next: Nature Nanotechnology


 Academic Frontier
Academic Frontier
