Science: Biocompatible hydrogel with multi-vascular network and functional intravascular topology
QQ Academic Group: 1092348845
Detailed

1. Article overview
Physical organs transport fluids through different vascular networks, which are biophysically and biochemically entangled to form complex three-dimensional (3D) transmission mechanisms, which are still difficult to produce and study. In this paper, food dye additives are used as light absorbers for projection stereolithography, and free intravascular structures are established through photopolymerization of hydrogels and multi-vascular structures are designed. This article shows the overall transparent hydrogel produced in a few minutes, including an efficient intravascular 3D fluid mixer and a functional mitral valve. This article further elaborates the entangled vascular network from the space-filling mathematical topology, and explores the oxygenation and flow of human red blood cells during tidal ventilation and proximal airway dilation. In addition, this article deployed a structured biodegradable hydrogel carrier in a rodent model of chronic liver injury to highlight the potential transformational utility of this material innovation.
Two, graphic guide
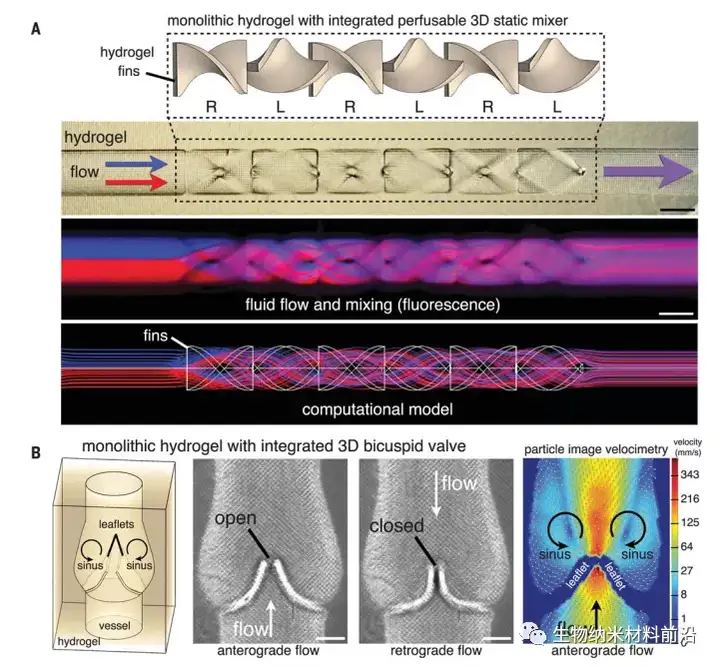
Figure 1. Hydrogel with functional intravascular topology; (A) Monolithic hydrogel with perfusible channels, which contains static elements, which quickly promote fluid splitting and mixing (as shown in fluorescence imaging), Consistent with the flow calculation model (scale bar, 1 mm); (B) Hydrogel that integrates the functional 3D mitral valve into the vessel wall under antegrade and retrograde flow (scale bar, 500 μm), particle image velocimetry A stable mirror image vortex appears in the sinus area behind the opened valve leaflets.Figure 1. Hydrogel with functional intravascular topology; (A) Monolithic hydrogel with perfusible channels, which contains static elements, which quickly promote fluid splitting and mixing (as shown in fluorescence imaging), Consistent with the flow calculation model (scale bar, 1 mm); (B) Hydrogel that integrates the functional 3D mitral valve into the vessel wall under antegrade and retrograde flow (scale bar, 500 μm), particle image velocimetry A stable mirror image vortex appears in the sinus area behind the opened valve leaflets.
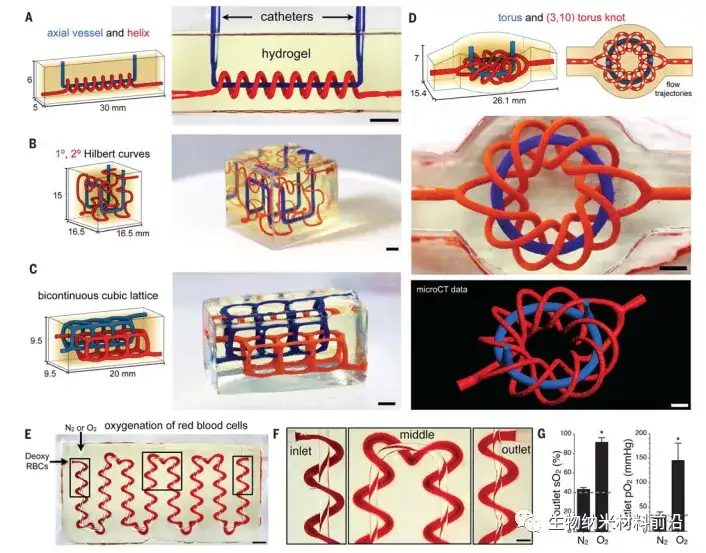
Figure 2. Entangled blood vessel network (A to D); the adaptability of mathematical space filling curve to the topology of entangled blood vessels in hydrogel (20 wt% PEGDA, 6 kDa): (A) axial blood vessels and spirals; (B) ) Interpenetrating Hilbert curve; (C) bicontinuous cubic lattice and (D) torus and (3,10) torus knot (scale bar, 3 mm); (E) inlay of axial blood vessel and its edge The winding spiral of a serpentine path. The photo is a top-down view of the manufactured hydrogel, where oxygen and red blood cells are delivered to various blood vessels. During the perfusion process, the color of red blood cells changed from dark red (at the red blood cell entrance) to bright red (at the red blood cell exit) (scale bar, 3 mm); the boxed area was enlarged in (F) (scale bar, 1 mm); (G) Collect the perfused RBC at the exit and quantify SO2 and PO2. Compared with deoxygenated red blood cells perfused at the entrance (dotted line) and nitrogen flow negative control (N ≥ 3 repetitions, data are mean ± SD, *P <2 × 10−7, Student t test).
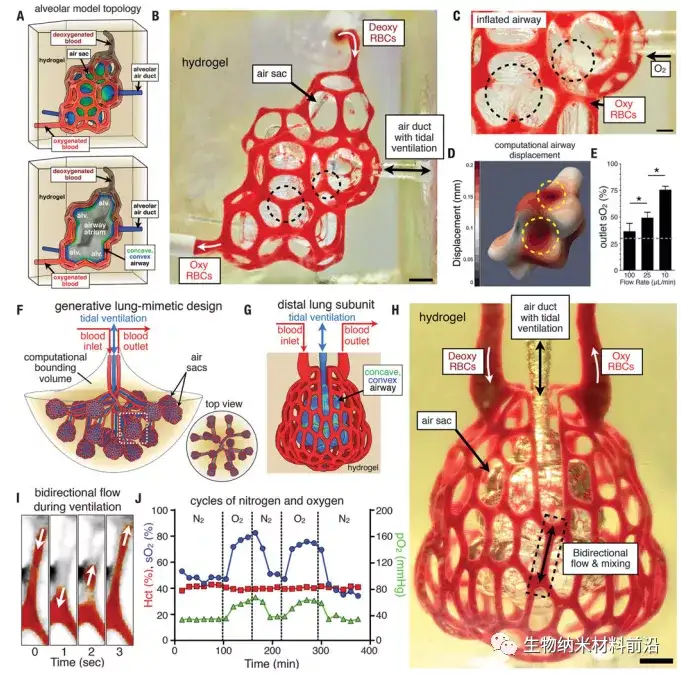
Figure 3. Tidal ventilation and oxygenation in a hydrogel with a vascularized alveolar model topology; (A) Alveolar model topology design based on Weaire-Phelan 3D mosaic and topology migration to derive the sheath system, cross-sectional view The model alveoli (alv.) and the shared airway atria are shown. Highlight the convex (blue) and concave (green) areas of the airway; (B) the photo of the hydrogel printed during the RBC perfusion process, while the balloon is ventilated with O2 (scale bar, 1 mm); (C) oxygen is used to make the gas After the airway is inflated, the concave area of the airway (black dashed circle) squeezes adjacent blood vessels and leads to the removal of red blood cells (scale bar, 500 μm); (D) The calculation model of the airway expansion shows that the displacement of the concave area increases (yellow dashed line) Circle); (E) The oxygen saturation of RBC increases with the decrease of RBC flow rate (N = 3, data is average ± SD, P <9 × 10−4 through Students t test), the dotted line indicates at the entrance SO2 of perfused deoxygenated RBC; (F) Lung simulation design is formulated through generative growth of airways, offset growth of opposite inlet and outlet vascular networks, and branch tip populations with distal lung subunits; (G) The distal lung subunit is composed of a concave-convex airway wrapped in the vasculature by 3D migration and anisotropic Voronoi mosaic; (H) a photo of a printed hydrogel containing the distal lung subunit during RBC perfusion, while The balloon is ventilated with O2 (scale bar, 1 mm) (I) The threshold view of the area enclosed by the dashed box in (H) shows the bidirectional RBC flow during ventilation; (J) The distal lung subunit can withstand more than 10,000 stably Four cycles of ventilation (24 kPa, 0.5 Hz), and show the sensitivity of RBC to ventilation gas (N2 or O2).
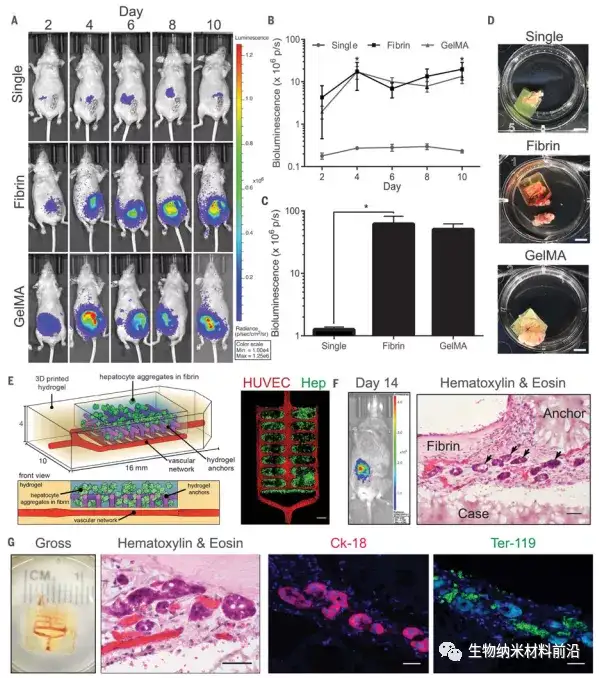
Figure 4. Implantation of a functional liver hydrogel carrier; (A to C) Albumin promoter activity was enhanced in a hydrogel carrier containing liver aggregates after implantation in nude mice, all time points for each condition The data is shown in (B) [N = 4, P <0.05]; (D) Gross image of the hydrogel after resection (scale bar, 5 mm); (E) (Left) Prevascularized liver hydrogel The vector was created by seeding endothelial cells (HUVEC) in the vascular network after printing. (right) Confocal microscopy showed that the hydrogel anchored and physically trapped the fibrin gel containing hepatocyte aggregates (Hep) ( Scale bar, 1 mm); (F) The hepatocytes in the vascularized liver hydrogel carrier showed albumin promoter activity after implantation in mice with chronic liver injury. The transplanted sections stained with H&E showed liver aggregates (black arrows) ) Positioning of printed (case, anchor) and non-printed (fibrin) components relative to the carrier system (scale bar, 50 μm); (G) The hydrogel carrier is infiltrated by host blood (total, H&E). The vector contains aggregates expressing the marker cytokeratin-18 (Ck-18) and is very close to Ter-119-positive RBC (scale bar, 40 μm).
3. Full text summary
This paper determines that synthetic and natural food dyes widely used in the food industry can be used as effective biocompatible light absorbers, which can realize the stereolithography production of hydrogels containing complex and functional vascular structures. In this paper, a light absorber is selected in food additives, the absorption spectrum of which contains visible light wavelengths that can be used for biocompatible photopolymerization, and the use of hydrogel pre-solutions containing lemon yellow, curcumin or anthocyanins have been prepared with certain Structure of hydrogel. In addition to these organic molecules, inorganic gold nanoparticles (50 nm) are widely recognized for their biocompatibility and light attenuation properties, and can also be used as an effective light-absorbing additive to generate infused hydrogels. In this paper, food dyes are used as light absorbers to produce hydrogels containing functional vascular topological structures, which are used to study fluid mixers, valves, inter-vascular transport, nutrient delivery, and host transplantation. Through stereolithography, it is possible Synchronous and orthogonal control of tissue structure and biological materials to design regeneration tissue.
This paper determines that synthetic and natural food dyes widely used in the food industry can be used as effective biocompatible light absorbers, which can realize the stereolithography production of hydrogels containing complex and functional vascular structures. In this paper, a light absorber is selected in food additives, the absorption spectrum of which contains visible light wavelengths that can be used for biocompatible photopolymerization, and the use of hydrogel pre-solutions containing lemon yellow, curcumin or anthocyanins have been prepared with certain Structure of hydrogel. In addition to these organic molecules, inorganic gold nanoparticles (50 nm) are widely recognized for their biocompatibility and light attenuation properties, and can also be used as an effective light-absorbing additive to generate infused hydrogels. In this paper, food dyes are used as light absorbers to produce hydrogels containing functional vascular topological structures, which are used to study fluid mixers, valves, inter-vascular transport, nutrient delivery, and host transplantation. Through stereolithography, it is possible Synchronous and orthogonal control of tissue structure and biological materials to design regeneration tissue.
Article link:
Doi:10.1126/science.aav9750.
Doi:10.1126/science.aav9750.
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
- Previous: Cryst. Growth Des.: Th
- Next: IF 16.9! Self-assemble


 Academic Frontier
Academic Frontier