Li Shiying/Cheng Hong/Yu Xiyong ACS Nano: Self-delivered photoimmunostimulant to achieve photodynamic sensitization tumor immunotherapy
QQ Academic Group: 1092348845
Detailed

Self-delivery of photosensitizers and immunomodulators to tumor sites can improve photodynamic immunotherapy, but this strategy is still challenging. Here, Professor Li Shiying, Professor Yu Xiyong of Guangzhou Medical University and Associate Professor Cheng Hong of Southern Medical University jointly reported a self-delivery photoimmunostimulant (called iPS) for photodynamic sensitization tumor immunotherapy.
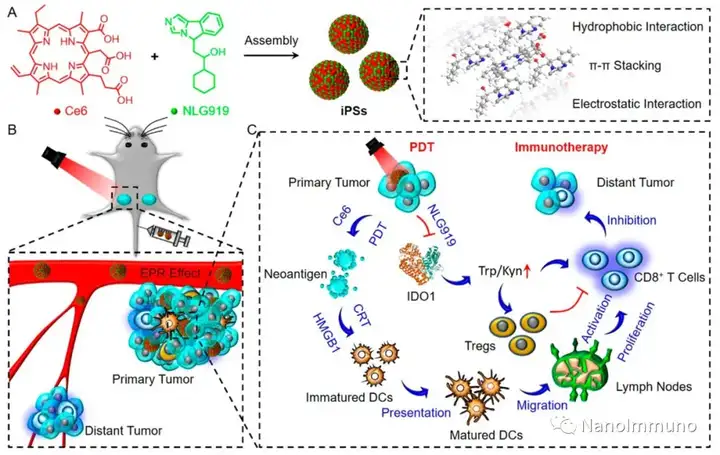
The carrier-free iPS is self-assembled through the non-covalent interaction between the photosensitizer Ce6 and NLG919, which avoids the toxicity and immunogenicity caused by excipients. After intravenous injection, iPS tends to passively accumulate in the tumor site to trigger powerful photodynamic therapy (PDT), induce immunogenic cell death (ICD), activate cytotoxic T lymphocytes (CTL) and initiate anti-tumor immune response. At the same time, the co-delivery of NLG919 inhibited the activation of indoleamine 2,3-dioxygenase 1 (IDO-1) and reversed the immunosuppressive tumor microenvironment. The photodynamic sensitization immunotherapy based on iPS can effectively inhibit the growth of primary and distal tumors, and has low system toxicity, which has inspired the development of clinically transformable and self-deliverable nanomedicines for precise tumor treatment.
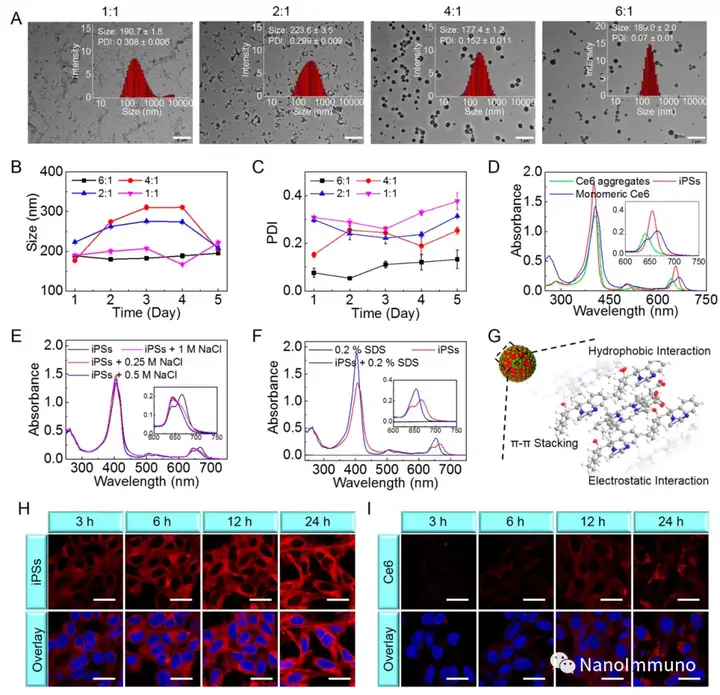
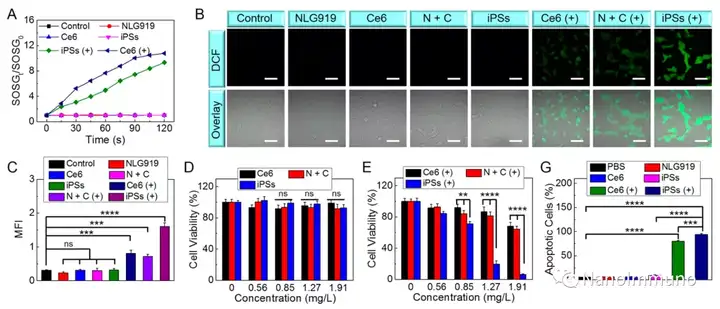
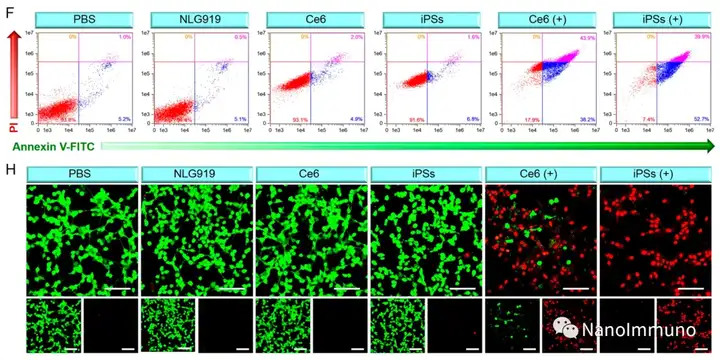
In this paper, self-delivery photoimmunostimulants (iPS) for photodynamic enhancement of tumor immunotherapy were prepared by self-assembly of photosensitizers Ce6 and NLG919. Due to hydrophobicity, π-π stacking and electrostatic interaction, self-assembled iPS has improved solubility, stability and biocompatibility. In addition, carrier-free iPS has a high drug loading rate, which can avoid potential immunogenicity. Interestingly, the powerful PDT treatment of iPS can not only trigger tumor cell apoptosis, but also trigger an ICD response by promoting the migration of calreticulin to the cell surface and the extracellular release of high-mobility group protein B1. Mature DCs will recruit and activate CTLs, realizing synergistic immunotherapy of primary and distal tumors. The co-delivery of NLG919 inhibited IDO-1 activation and Treg tumor invasion, thereby reversing the immunosuppressive tumor microenvironment.
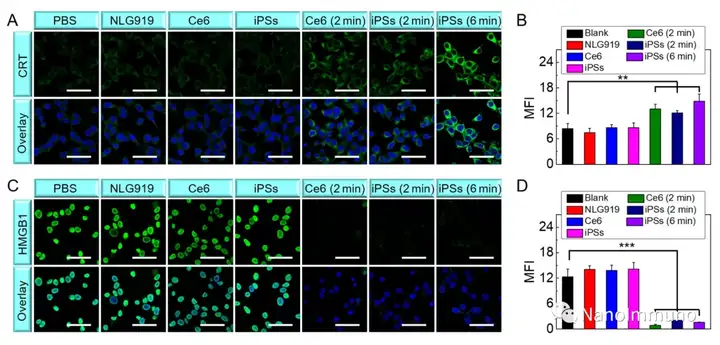
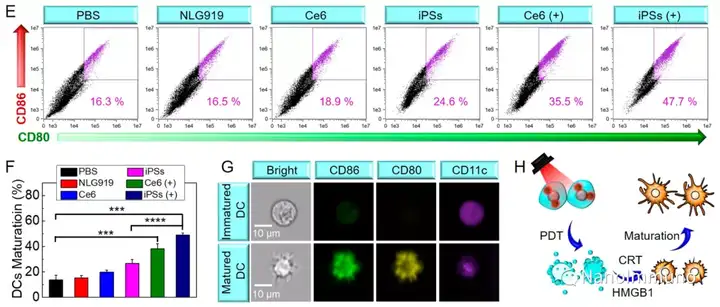
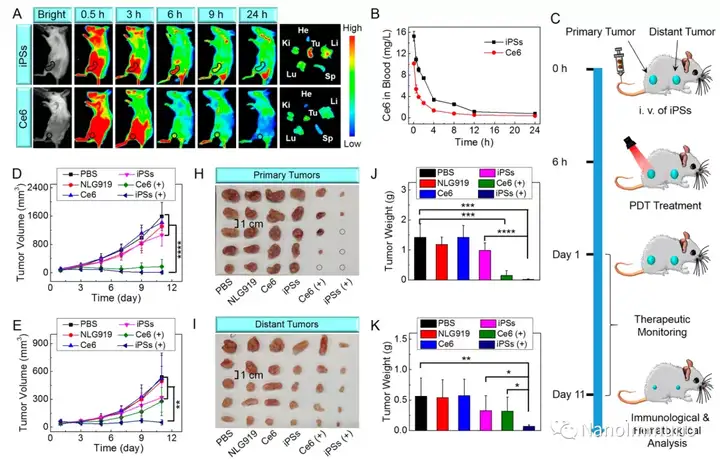
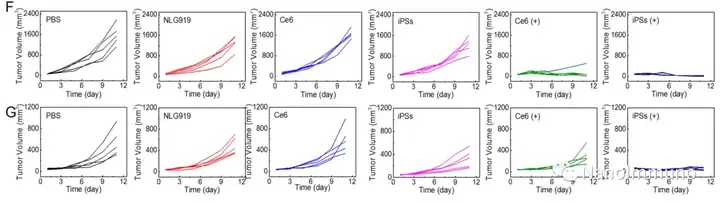
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
- Previous: The latest development
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier