Artificial Nano Red Blood Cells: Drug Delivery Carriers to Improve the Curative Effect of Chemotherapy
QQ Academic Group: 1092348845
Detailed
Research Background
Tumor immunotherapy has been widely studied in clinical trials in recent years because of its high curative effect and low side effects. However, immune response preparations usually require high doses to ensure immunotherapeutic effects, which will inevitably cause a series of side effects such as systemic immune activation. In recent years, many scientific researchers have tried to use nanosystems for the delivery of immune response preparations. However, the safety and toxicity of inorganic carriers used to construct nanosystems still have some challenges that need to be overcome. Therefore, in order to promote the clinical transformation of nano-delivery system related research as soon as possible, the use of clinically approved materials may be a viable choice for various tumor models in the future.
Tumor immunotherapy has been widely studied in clinical trials in recent years because of its high curative effect and low side effects. However, immune response preparations usually require high doses to ensure immunotherapeutic effects, which will inevitably cause a series of side effects such as systemic immune activation. In recent years, many scientific researchers have tried to use nanosystems for the delivery of immune response preparations. However, the safety and toxicity of inorganic carriers used to construct nanosystems still have some challenges that need to be overcome. Therefore, in order to promote the clinical transformation of nano-delivery system related research as soon as possible, the use of clinically approved materials may be a viable choice for various tumor models in the future.
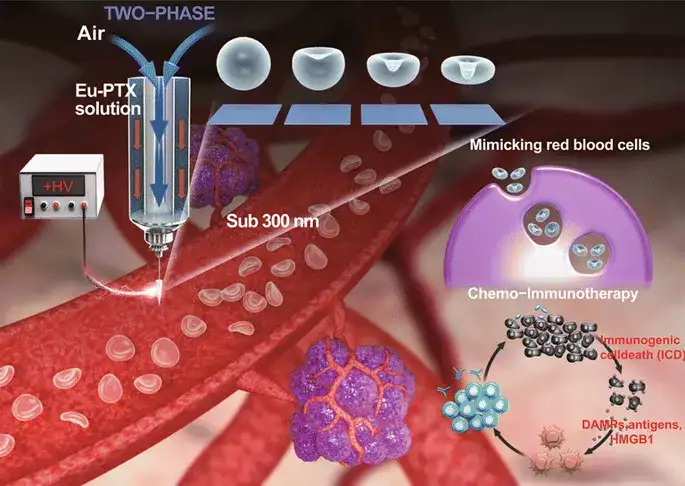
Artificial Nanoscale Erythrocytes from Clinically Relevant Compounds for Enhancing Cancer ImmunotherapyWenquan Ou, Kang Sik Nam, Dae Hoon Park, Jungho Hwang*, Sae Kwang Ku, Chul Soon Yong, Jong Oh Kim*, Jeong Hoon Byeon*Nano‑Micro Lett.(2020) 12:90https://doi.org/10.1007/s40820‑020‑00428‑y
Highlights of this article
1. A two-coaxial electrospray method was designed to prepare paclitaxel-loaded pseudo-hemocyte particles. 2. The combined anti-programmed death ligand 1 antibody (Eu-FBCP/PTX+aPL) can further improve the efficacy of chemotherapy and immunity.
brief introduction
Currently, the clinically approved polymer pharmaceutical resin-polyacrylic resin (Eudragit®[Eu]), as a biomimetic nanosystem, can effectively carry antibodies (aPL) against programmed death ligand (PD-L1). This research has developed a gas-liquid two-phase electrospray. When the mechanical and electrical parameters of the spray are balanced, it can continuously produce a biomimetic nanosystem composed of only clinically relevant compounds [paclitaxel-loaded pseudo-blood cell polyacrylic resin ( Eu) particles (Eu-FBCP/PTX)], this system provides a conceptual leap for the development of clinically transformed chemical-immunotherapy nanosystems. The Eu-FBCP/PTX nanosystem exhibits phagocytosis and large pinocytosis cell uptake; in the absence and presence of anti-PD-L1 antibodies, it is more effective than similar-sized PTX-loaded spherical Eu particles (Eu-s/PTX). It has better curative effect in chemo-immunotherapy.
Graphic guide
I Preparation and characterization
The bubble compression method is used to generate the concave structure of Eu-FBCP/PTX. The designed two-phase electrospray successfully prepared the concave structure, which means that the bubble pressing of the dried Eu solute can overcome the viscous force and surface tension (better to form a spherical shape) to produce an anisotropic shape. Incubate MC-38 and B16BL/6 tumor cells with Cy5.5-labeled nanosystems to detect the difference in cell uptake between Eu-FBCP/Cy5.5 and Eu-s/Cy5.5. The results showed that the cellular uptake of the Cy5.5-labeled Eu(RL) nanosystem in MC-38 and B16BL/6 cells was significantly lower than that of Eu-FBCP/Cy5.5 or Eu-s/Cy5.5 quantity. The average particle size of Eu-FBCP/PTX is 320.8 nm. The dispersion of Eu-FBCP/PTX nanosystem within 48 hours has a time-dependent slow-release characteristic (matching HIGUCHI model).
1. A two-coaxial electrospray method was designed to prepare paclitaxel-loaded pseudo-hemocyte particles. 2. The combined anti-programmed death ligand 1 antibody (Eu-FBCP/PTX+aPL) can further improve the efficacy of chemotherapy and immunity.
brief introduction
Currently, the clinically approved polymer pharmaceutical resin-polyacrylic resin (Eudragit®[Eu]), as a biomimetic nanosystem, can effectively carry antibodies (aPL) against programmed death ligand (PD-L1). This research has developed a gas-liquid two-phase electrospray. When the mechanical and electrical parameters of the spray are balanced, it can continuously produce a biomimetic nanosystem composed of only clinically relevant compounds [paclitaxel-loaded pseudo-blood cell polyacrylic resin ( Eu) particles (Eu-FBCP/PTX)], this system provides a conceptual leap for the development of clinically transformed chemical-immunotherapy nanosystems. The Eu-FBCP/PTX nanosystem exhibits phagocytosis and large pinocytosis cell uptake; in the absence and presence of anti-PD-L1 antibodies, it is more effective than similar-sized PTX-loaded spherical Eu particles (Eu-s/PTX). It has better curative effect in chemo-immunotherapy.
Graphic guide
I Preparation and characterization
The bubble compression method is used to generate the concave structure of Eu-FBCP/PTX. The designed two-phase electrospray successfully prepared the concave structure, which means that the bubble pressing of the dried Eu solute can overcome the viscous force and surface tension (better to form a spherical shape) to produce an anisotropic shape. Incubate MC-38 and B16BL/6 tumor cells with Cy5.5-labeled nanosystems to detect the difference in cell uptake between Eu-FBCP/Cy5.5 and Eu-s/Cy5.5. The results showed that the cellular uptake of the Cy5.5-labeled Eu(RL) nanosystem in MC-38 and B16BL/6 cells was significantly lower than that of Eu-FBCP/Cy5.5 or Eu-s/Cy5.5 quantity. The average particle size of Eu-FBCP/PTX is 320.8 nm. The dispersion of Eu-FBCP/PTX nanosystem within 48 hours has a time-dependent slow-release characteristic (matching HIGUCHI model).
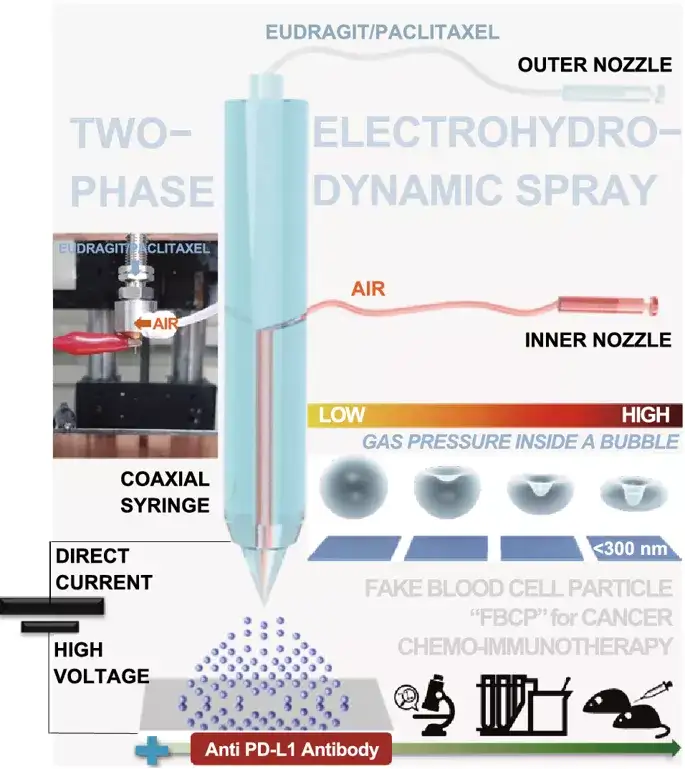
Figure 1. Air (inner nozzle)-liquid (outer nozzle; Eu/PTX in ethanol) two-phase electrospray diagram. It can use the Eu/PTX melt to pressurize the bubbles in the dry droplets to generate concave (imitating the shape of blood cells) particles (Eu FBCP/PTX) under the balance between electrical (BE) and mechanical (CaRe) parameters. In combination with anti-programmed death ligand 1 (PD-L1) antibody (aPL) chemoimmunotherapy. The use of single-phase (only for Eu/PTX solutions) electrospray produces spherical particles of similar size (Eu-s/PTX) for comparison in the absence of aPL.
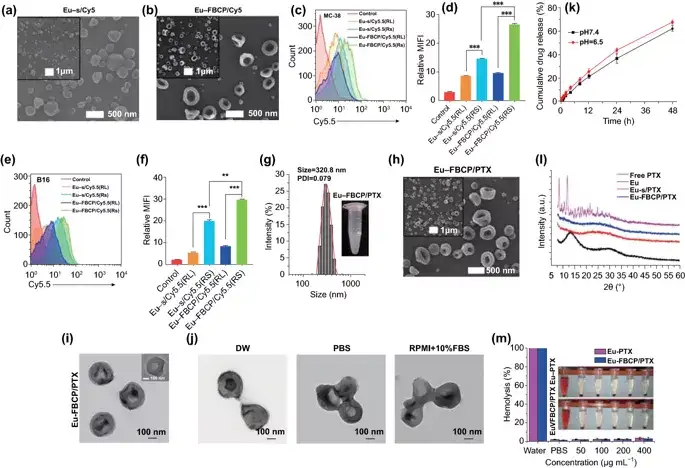
Figure 2. Characterization of Eu-FBCP and Eu-s in the absence of PTX. a, b High and low scanning electron microscope images of Eu-s/Cy5.5 and Eu-FBCP/Cy5.5. By controlling the concentration of Eu in the ethanol solution, the Eu-s/Cy5.5 of similar size was prepared without spraying air into the inner nozzle. cf FACS results (n=3; fluorescence curve and average fluorescence intensity [MFI]) were used to compare the MC-38(c,d) or B16(e) between Eu-FBCP and Eu-s after Cy5.5 was added to electrospray , F) Cell uptake to check that Eu-FBCP has better uptake due to its concave shape. The analysis includes Eu-RL to confirm the difference in absorption between Eu-RS and Eu-RL and provide a basis for choosing Eu-RS. g DLS size distribution and PDI of Eu-FBCP/PTX in PBS (embedded digital image). h,i High and low power scanning electron microscope (h) and transmission electron microscope (i) images of Eu-FBCP/PTX. j Typical TEM image of Eu-FBCP/PTX dispersed in DW, PBS or RPMI+10% FBS for 8 hours to study the hydrodynamic stability of different media. k Release PTX from Eu-FBCP/PTX for 48 hours at pH 6.5 or pH 7.4 (n=3). i The XRD spectra of Eu-FBCP/PTX and Eu-s/PTX and free PTX and Eu (RS; before electrospray) to investigate and compare the incorporation of Eu and PTX. m Use different concentrations of Eu-FBCP/PTX or Eu-s/PTX (50-400μg mL-1) to culture red blood cells for 8 hours after hemolysis results (n=3; **p<0.01 and **p< 0.001).
II In vitro cell viability and cell uptake
MTT results showed that both Eu-FBCP and Eu-s (in the absence of PTX) showed high cell viability (>95%), while free PTX induced dose-dependent cytotoxicity, showing a maximum of 28.4μg mL-1 The median inhibitory concentration (IC50). The maximum half inhibitory concentrations of Eu-s/PTX and Eu-FBCP/PTX were 15.9 and 9.7μg mL-1, respectively, which were significantly lower than those of free PTX. Studies have shown that the cellular uptake of Eu-FBCP/Cy5.5 is 3 times higher than that of Eu-s/Cy5.5 and Eu-FBCP/Cy5.5 enters cells mainly through phagocytosis and macropinocytosis.
II In vitro cell viability and cell uptake
MTT results showed that both Eu-FBCP and Eu-s (in the absence of PTX) showed high cell viability (>95%), while free PTX induced dose-dependent cytotoxicity, showing a maximum of 28.4μg mL-1 The median inhibitory concentration (IC50). The maximum half inhibitory concentrations of Eu-s/PTX and Eu-FBCP/PTX were 15.9 and 9.7μg mL-1, respectively, which were significantly lower than those of free PTX. Studies have shown that the cellular uptake of Eu-FBCP/Cy5.5 is 3 times higher than that of Eu-s/Cy5.5 and Eu-FBCP/Cy5.5 enters cells mainly through phagocytosis and macropinocytosis.
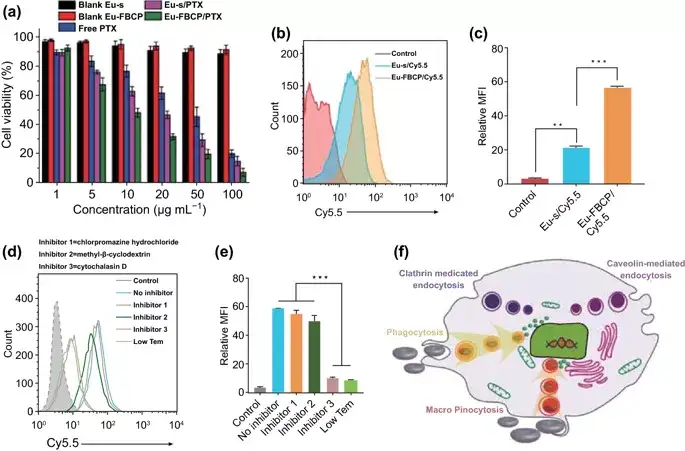
Figure 3. In vitro bioassay results and possible mechanism of Eu-FBCP/PTX treatment of MC-38 cells. (**p<0.01 and **p<0.001). a Treat MC-38 cells with Eu-FBCP/PTX or Eu-s/PTX for 24 hours, as well as free PTX and single Eu-FBCP or Eu-s (n=6). b, c Compare the FACS results of Eu-FBCP/Cy5.5 and Eu-s/Cy5.5 cell uptake (n=3; fluorescence spectrum and MFI). d, e FACS results (n=3; fluorescence spectrum and MFI) were used to compare the effects of different inhibitors (including low temperature conditions) pretreatment on the uptake of Eu-FBCP/Cy5.5 cells. f Schematic diagram of the uptake pathway of cells pretreated with different inhibitors and subsequently incubated with Eu-FBCP/Cy5.5.
III Cell Cycle and Apoptosis
Eu-FBCP/PTX induced 85.4% of cell apoptosis (early and late: Q2+Q3), which was 16.8% higher than Eu-s/PTX. The loss of mitochondrial membrane potential (ΔΨm) is an important sign of Cyt c transport from mitochondria to cytoplasm, and is the beginning of the apoptosis program. The Eu-FBCP/PTX nanosystem has a stronger mitochondrial damage effect than other drug groups.
III Cell Cycle and Apoptosis
Eu-FBCP/PTX induced 85.4% of cell apoptosis (early and late: Q2+Q3), which was 16.8% higher than Eu-s/PTX. The loss of mitochondrial membrane potential (ΔΨm) is an important sign of Cyt c transport from mitochondria to cytoplasm, and is the beginning of the apoptosis program. The Eu-FBCP/PTX nanosystem has a stronger mitochondrial damage effect than other drug groups.
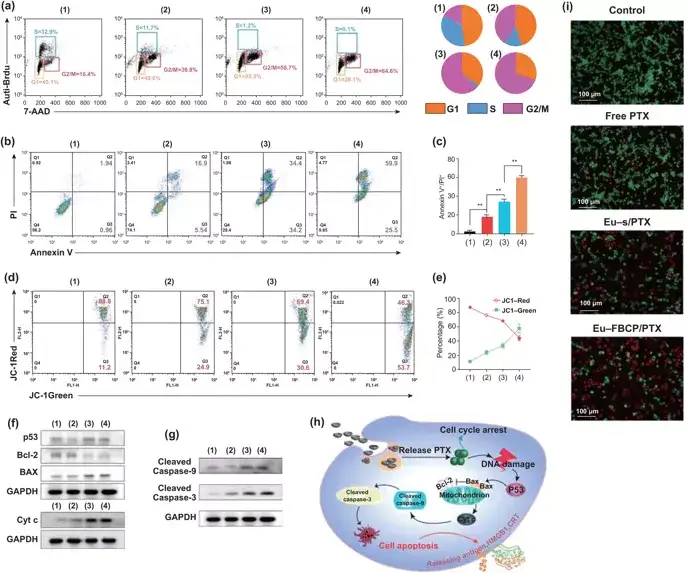
Figure 4. In vitro cell cycle and apoptosis analysis of different groups of MC-38 cells (1: control, 2: free PTX, 3: Eu-s/PTX, 4: Eu-FBCP/PTX). a Cell cycle analysis results of different treatments (1-4). Brdu and PI are used to calculate the cell fraction of each phase (G1, S or G2/M) in the cell cycle. b, c Use Annexin V-FITC/PI kit (n=3) to analyze the apoptosis and quantitative data of 24-hour treatment (1-4). d, e After 24 hours of treatment, the FACS curve of the cells and the quantitative data of JC-1 staining showed the change of the mitochondrial membrane potential of (1-4) cells (n=3). f,g Representative expression of p53, Bcl-2, Bax, Cyt-c, lysed caspase-9 and lysed caspase-3 in therapeutic cells (1-4). h Schematic diagram of the possible mechanism of apoptosis of therapeutic cells (1-4) based on expression (f, g). i Representative microscope images of AO/PI stained cells in live/dead cell experiments after treatment (1-4).
IV Immune response induced by immunogenic cell death
PTX can induce immunogenic cell death (ICD) from the surface of dead cells by releasing tumor antigens, such as HMGB1, CRT and ATP. The secretion of HGMB1 and CRT is the key factor that promotes the maturation of dendritic cells (DCs) and triggers ICD. After treating the cells with Eu-FBCP/PTX, the expression of HMGB1 and CRT was much higher than that of Eu-s/PTX group or free PTX group. These molecules acted as auxiliary stimuli for DC maturation and induced 85.6% of mature DCs, 21.9% more mature DCs than Eu-s/PTX (63.7%) and 2 times more mature DCs than free PTX (42.3%). above. These results show that Eu-FBCP/PTX treatment cells can maximize the expression of CRT and HMGB1, promote the maturation of DCs and the activation of IFN-γ+CD8+ T cells.
IV Immune response induced by immunogenic cell death
PTX can induce immunogenic cell death (ICD) from the surface of dead cells by releasing tumor antigens, such as HMGB1, CRT and ATP. The secretion of HGMB1 and CRT is the key factor that promotes the maturation of dendritic cells (DCs) and triggers ICD. After treating the cells with Eu-FBCP/PTX, the expression of HMGB1 and CRT was much higher than that of Eu-s/PTX group or free PTX group. These molecules acted as auxiliary stimuli for DC maturation and induced 85.6% of mature DCs, 21.9% more mature DCs than Eu-s/PTX (63.7%) and 2 times more mature DCs than free PTX (42.3%). above. These results show that Eu-FBCP/PTX treatment cells can maximize the expression of CRT and HMGB1, promote the maturation of DCs and the activation of IFN-γ+CD8+ T cells.
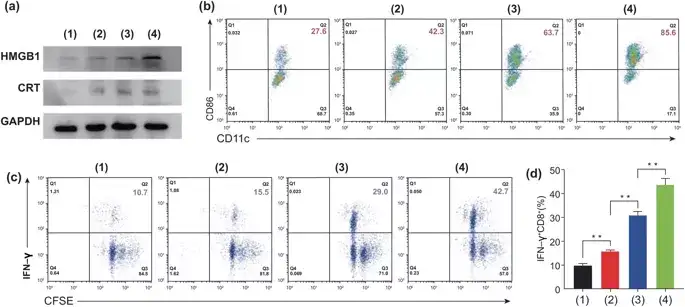
图5. After treatment with different methods, conduct in vitro bioassays on MC-38 cells to detect the DC cell maturation and CD8+ T cell activation induced by ICD. 1: control group, 2: free PTX, 3: Eu-s/PTX and 4: Eu-FBCP/PTX). a The representative expression of HMGB1 and CRT in the treatment group (1-4), showing the induction of ICD. b After treating the cells (1–4) CD11c+–CD86+ (as an indicator of DC maturity) recognizes the maturity of DCs through the TAA produced. c CFSE-IFN-γ detects the proliferation and activation of CD8+ T cells. d After treating the cells (1-4) activated IFN-γ+CD8+ T cells.
V Distribution in the body
Within 12 hours of intravenous injection of mice, the fluorescence intensity of the tumor site gradually increased, and after 24 hours, the fluorescence intensity began to decrease. Among them, the fluorescence intensity of the tumor site in the Eu-FBCP/Cy5.5 group was 1.76 times that of the Eu-s/Cy5.5 group .
V Distribution in the body
Within 12 hours of intravenous injection of mice, the fluorescence intensity of the tumor site gradually increased, and after 24 hours, the fluorescence intensity began to decrease. Among them, the fluorescence intensity of the tumor site in the Eu-FBCP/Cy5.5 group was 1.76 times that of the Eu-s/Cy5.5 group .
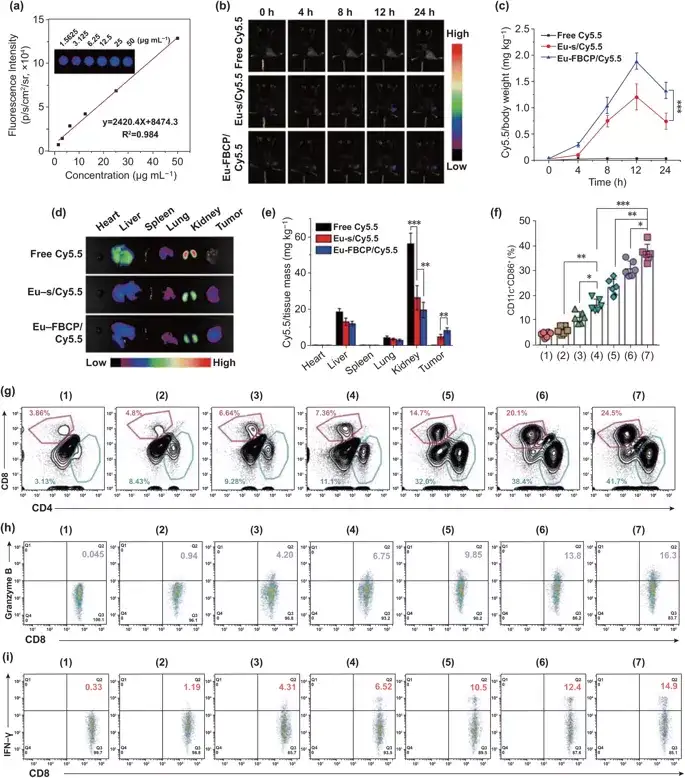
Figure 6. Eu-FBCP/Cy5.5 (or Eu-s/Cy5.5) in vivo biodistribution and different treatments (1: PBS, 2: free PTX, 3: Eu-s/PTX, 4: Eu-FBCP /PTX, 5: aPL, 6: Eu-s/PTX+aPL, 7: Eu-FBCP/PTX+aPL) anti-tumor immune response. a After in vivo treatment, use an animal imaging system to obtain a calibration curve of fluorescence Cy5.5 with a concentration range of 1.5625 to 50.0000 μg/ml. b Fluorescence time imaging (0, 4, 8, 12 and 24 hours) to detect the tumor accumulation of Eu-FBCP/Cy5.5, Eu-s/Cy5.5 and free Cy5.5 after intravenous injection. c The distribution of Cy5.5 in the tumor at different time points. d,e Typical fluorescence images and quantitative data of mouse major organs and tumors 24 hours after intravenous injection. f Different treatment methods are intravenously injected into dendritic cell tumors to mature (1-7) (n = 6; *p< 0.05, **p< 0.01, and ***p< 0.001). g CD4+–CD8+ T cells infiltrate the tumor microenvironment after drug treatment (1-7). g, i The level of CD8+ T cells CD8+–granzyme B+ and CD8+–IFN-γ+ in the tumor after treatment with drugs (1-7).
VI Anti-tumor immunotherapy in vivo
In order to confirm the important role of Eu-FBCP/PTX nanosystem in ICD-induced immunotherapy, a related in vivo model was further constructed to detect the maturation and anti-tumor IFN-γ+ of DCs in the absence and presence of aPL. CD8+ T cell activation. Experimental results show that EuFBCP/PTX-induced ICD can enhance the anti-tumor immune response of aPL and induce a large number of activated CD8+ T cells to fight against MC-38 tumors. VII Combined anti-tumor effect in vivo Inspired by the combination of Eu-FBCP/PTX and aPL to activate the anti-tumor immune response, the efficacy of combined therapy was evaluated on MC-38 tumor-bearing immunocompetent C57BL/6 mice. In the absence of aPL, Eu-FBCP/PTX and Eu-s/PTX can improve the efficacy of PTX and moderately inhibit tumor growth. Eu-FBCP/PTX + aPL can significantly inhibit the growth of tumors, and it has basically no effect on the weight of mice after administration. It has a longer life span than mice in other experimental groups, confirming the better curative effect of this nanosystem And biological safety. Further experiments verified the important role of ICD induction and anti-tumor immune response in the treatment of this system.
VI Anti-tumor immunotherapy in vivo
In order to confirm the important role of Eu-FBCP/PTX nanosystem in ICD-induced immunotherapy, a related in vivo model was further constructed to detect the maturation and anti-tumor IFN-γ+ of DCs in the absence and presence of aPL. CD8+ T cell activation. Experimental results show that EuFBCP/PTX-induced ICD can enhance the anti-tumor immune response of aPL and induce a large number of activated CD8+ T cells to fight against MC-38 tumors. VII Combined anti-tumor effect in vivo Inspired by the combination of Eu-FBCP/PTX and aPL to activate the anti-tumor immune response, the efficacy of combined therapy was evaluated on MC-38 tumor-bearing immunocompetent C57BL/6 mice. In the absence of aPL, Eu-FBCP/PTX and Eu-s/PTX can improve the efficacy of PTX and moderately inhibit tumor growth. Eu-FBCP/PTX + aPL can significantly inhibit the growth of tumors, and it has basically no effect on the weight of mice after administration. It has a longer life span than mice in other experimental groups, confirming the better curative effect of this nanosystem And biological safety. Further experiments verified the important role of ICD induction and anti-tumor immune response in the treatment of this system.
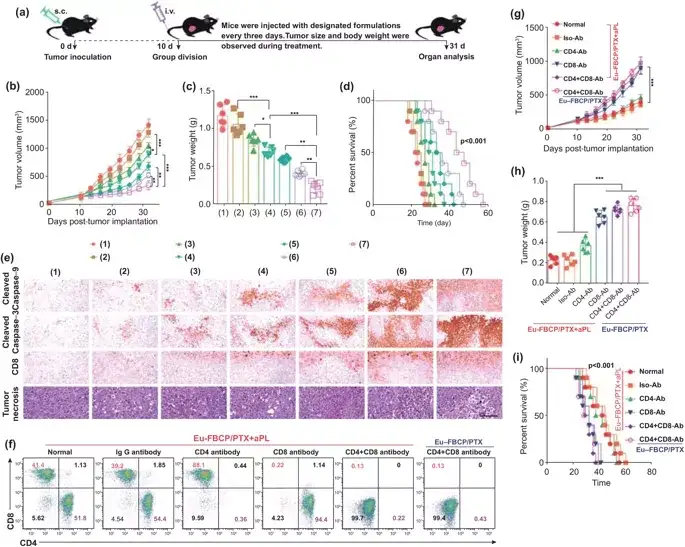
Figure 7. The anti-tumor effects of Eu-FBCP/PTX and Eu-s/PTX in the absence of aPL (inducing chemical ICD) and the presence (enhancing immune anti-tumor activity) in vivo (*p<0.05, **p<0.01, and ***p <0.001). a Different treatments (1: PBS, 2: free PTX, 3: Eu-s/PTX, 4: Eu-FBCP/PTX, 5: aPL, 6: Eu-s/PTX+aPL and 7: Eu-FBCP/PTX +aPL) Schematic diagram of the experimental schedule for in vivo anti-tumor research. b, c Time distribution of tumor size and final tumor weight collected from treated mice (1-7; 6 in each group). d Survival curve of treated mice (1-7; 10 mice in each group). e Histopathological and immunohistochemical expressions of cleaved caspase-9, cleaved caspase-3, CD8+ and tumor necrosis factor in tumor sections obtained from treated mice (1-7 mice, 6 mice in each group). f Immune cells are generated after pretreatment with PBS (normal), IgG antibody (Iso-Ab), CD4 antibody (CD4-Ab), CD8 antibody (CD8-Ab) and CD8+CD4 antibody (CD4+CD8-Ab), construct Immunohistochemistry MC-38 tumor-bearing mouse model. g, h Time distribution of tumor size and final tumor weight of immunohistochemical mice (6 in each group) treated with Eu-FBCP/PTX+aPL or Eu-FBCP/PTX alone. i Survival curve of immune complex mice treated with Eu-FBCP/PTX+aPL or Eu-FBCP/PTX alone (10 mice per group).
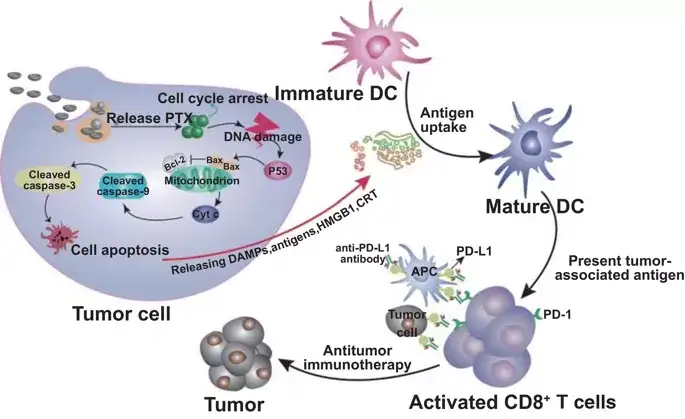
Figure 8. Schematic diagram of a reliable model for promoting efficacy from Eu-FBCP/PTX-induced ICD by combining Eu-FBCP/PTX with aPL. Eu-FBCP/PTX enters tumor cells through phagocytosis and macropinocytosis to release PTX, induce cell cycle G2/M phase and endogenous apoptosis. Apoptotic cancer cells release HMGB1 and CRT to promote DC maturation and CD8+ T cell activation. Eu-FBCP-PTX-induced ICD further promotes the curative effect of aPL, and its bionic performance is better than Eu-s/PTX.
Written by: "Nawei Express" Editorial Department
Editor: "Nawei Express" Editorial Department
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
Written by: "Nawei Express" Editorial Department
Editor: "Nawei Express" Editorial Department
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
- Previous: Korea National Cancer
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier