Energy Environ. Mater.|Charge Balancer in Accordion - MXene Modified Layer Induces Spherical Lithium Deposition
QQ Academic Group: 1092348845
Detailed
North Konami can provide MXene materials (customizable)
Research abstract
Solid-state lithium-ion batteries assembled with polymer electrolytes are currently the safest energy storage devices. However, conventional polymer electrolytes suffer from poor compatibility with lithium anodes and high interfacial impedance. Acetylene black (AB) and MXene have the advantages of high electrical conductivity. The anisotropic layered structure of MXene can provide more effective reaction interfaces and active sites during the redox reaction process, and improve the transport kinetics of ions and electrons. It is widely used in the field of secondary batteries. Therefore, we propose an interfacial modification method of coating an AB/MXene modified layer on the polymer electrolyte on the lithium anode side to solve the problem of interfacial compatibility and achieve the effect of suppressing lithium dendrites. The conductive AB and MXene in the interface modification layer can promote the uniform arrangement of electrons, induce the uniform deposition of lithium ions, and promote the generation of Li3N and LiF to form a dense solid electrolyte interface (SEI) layer, thereby improving the cycle performance and stability of the battery .
Introduction
Recently, the team of Professor Li Libo of Harbin University of Technology used a mixed conductive layer (MCL) prepared from AB/MXene to perform interfacial modification of solid-state lithium-ion batteries. Since the polymer electrolyte and lithium anode in solid-state lithium-ion batteries belong to solid-solid contact, the interface contact is poor and the lithium ion migration rate is low. The introduction of AB provides a soft interface for the polymer electrolyte and the lithium anode, solves the problem of poor interface contact, and achieves uniform charge and promotes uniform deposition of lithium ions through its own conductivity. The introduction of MXenes with unique accordion morphologies provides efficient charge transfer and lithium ion diffusion channels, accelerates the electrochemical reaction kinetics, induces the nucleation and growth of lithium ions on the surface of nanosheets, and effectively promotes the formation of polymer electrolyte- The lithium anode interface forms a uniform and dense interface layer, and the synergistic effect of AB and MXene improves the performance of the battery. The transport properties and charge distributions of AB and MXene were calculated using the DFTB+ module in Materials Studio. Lithium atoms are positively charged, while carbon atoms are negatively charged on the surface. The negative charge of MXene forms a charge band, which enables the uniform deposition of lithium ions and Effectively reduce the nucleation overpotential of lithium ions. More importantly, the MXene obtained after etching MAX with LiF and hydrochloric acid has a large number of −F ends, which can induce the formation of LiF (−Ti−F + Li+ + e−→−Ti + LiF) during cycling, and LiF is good. The high ionic conductivity and lower surface energy can facilitate reversible lithium ion transport and form a stable SEI film at the interface. After modification with AB and AB/MXene, respectively, the reversible capacity of the battery was greatly improved. The initial discharge specific capacities at 0.5 C cycle at room temperature are 111.1 mAh g-1 and 111.6 mAh g-1, respectively, and the discharge specific capacities after 100 cycles are 59.5 mAh g-1 and 91.2 mAh g-1, respectively. Even at a current density of 1 C, the battery with the AB/MXene modified layer can maintain a reversible capacity of 107.8 mAh g-1 with excellent cycling stability and rate performance.
The result was published online in the top international journal Energy & Environmental Materials (impact factor 13.443) with the title: A Charge Equalizer in Accordion—MXene-Modified Layer Leading to Spherical Lithium Deposition.
Zhao Yangmingyue is the first author of this article.
Graphical guide
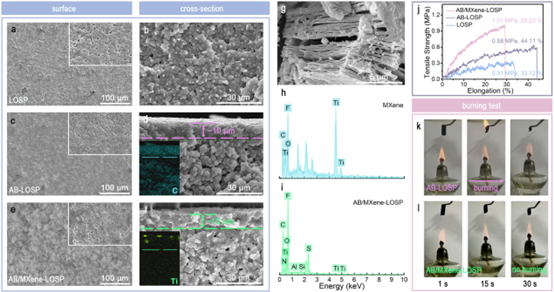
Figure 2. SEM images of surfaces (a, c, e) and cross-sections (b, d, f) of unmodified PE (LOSP), AB-LOSP, and AB/MXene-LOSP PEs and SEM images of MXene (g). EDAX spectra of MXene (h) and AB/MXene-LOSP (i), tensile strength curves (j), combustion experiments of AB-LOSP (k) and AB/MXene-LOSP (l). These excellent physical properties will be the prerequisites for polymer electrolyte LIBs with good safety and electrochemical performance.
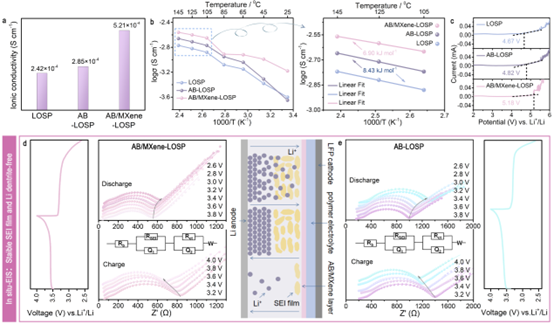
Figure 3. Ionic conductivity (a), effect of temperature on ionic conductivity (b), linear sweep voltammetry of LOSP, AB-LOSP, AB/MXene-LOSP PEs (c), AB/MXene-LOSP (d) ) and in situ EIS of AB-LOSP PEs (e). The SEI film induced by AB/MXene is beneficial to reduce the diffusion resistance of Li ions. The SEI film between the AB/MXene-LOSP electrolyte and the electrode is stable during battery cycling with good electrochemical performance.
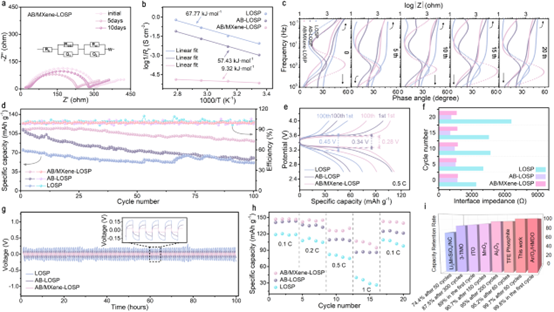
Figure 4. EIS of symmetric lithium batteries composed of AB/MXene-LOSP PEs at different storage times (a), the effect of temperature on the interfacial impedance of lithium electrode-PEs (b), LOSP, AB-LOSP, AB/MXene-LOSP EIS bode diagram of the assembled LIBs (c), charge-discharge (d) and voltage-capacity (e) curves of LIBs assembled with LOSP, AB-LOSP, and AB/MXene-LOSP PEs at 0.5 C and room temperature, with different cycles The interfacial impedance of the number of times (f), the galvanostatic polarization curve of a symmetric battery with a current density of 0.05 mA cm-2 (g), the cycling curves at different rates (h), the capacity of the MCL interface modified LIB is comparable to the interface modification reported in the literature. Comparison of capacity retention of sexual LIBs (i). The MCL layer forms a more stable SEI film, which effectively reduces the interface impedance and polarization voltage, thereby improving the cycle performance and rate performance of the battery.
Summarize
In this paper, the electrolyte-anode interface modification layer is utilized to promote the uniform deposition of Li ions. The ionic conductivity of AB/MXene-LOSP PE reaches 5.21×10-4 S cm-1. The LiFePO4 battery assembled from AB/MXene-LOSP PE achieved a capacity of 91.2 mAh g-1 with a Coulombic efficiency of 99.83% after 100 cycles at 0.5 C and room temperature. Li nucleation experiments verify that the AB/MXene interfacial modification layer improves the uniform deposition and redox kinetics of Li. Because MXene and AB can tune the current density through uniform charge transfer. Therefore, lithium dendrites are suppressed, while spherical lithium is induced. In situ EIS investigated the beneficial effect of the modified layer on SEI film formation. SEM and XPS showed that the addition of etched MXene produced a LiF-enriched interfacial layer. A uniform SEI layer at the interface can reduce the overpotential (0.04 V) and achieve the cycling stability of the battery. This work will inspire our development in inducing uniform deposition of lithium ions and lowering the lithium ion transport barrier at the electrolyte-anode interface.
Literature link
https://doi.org/10.1002/eem2.12463
For the original text, please click the lower left corner of the tweet to read the original text
Special thanks to the author of this article for their strong support


Research abstract
Solid-state lithium-ion batteries assembled with polymer electrolytes are currently the safest energy storage devices. However, conventional polymer electrolytes suffer from poor compatibility with lithium anodes and high interfacial impedance. Acetylene black (AB) and MXene have the advantages of high electrical conductivity. The anisotropic layered structure of MXene can provide more effective reaction interfaces and active sites during the redox reaction process, and improve the transport kinetics of ions and electrons. It is widely used in the field of secondary batteries. Therefore, we propose an interfacial modification method of coating an AB/MXene modified layer on the polymer electrolyte on the lithium anode side to solve the problem of interfacial compatibility and achieve the effect of suppressing lithium dendrites. The conductive AB and MXene in the interface modification layer can promote the uniform arrangement of electrons, induce the uniform deposition of lithium ions, and promote the generation of Li3N and LiF to form a dense solid electrolyte interface (SEI) layer, thereby improving the cycle performance and stability of the battery .
Introduction
Recently, the team of Professor Li Libo of Harbin University of Technology used a mixed conductive layer (MCL) prepared from AB/MXene to perform interfacial modification of solid-state lithium-ion batteries. Since the polymer electrolyte and lithium anode in solid-state lithium-ion batteries belong to solid-solid contact, the interface contact is poor and the lithium ion migration rate is low. The introduction of AB provides a soft interface for the polymer electrolyte and the lithium anode, solves the problem of poor interface contact, and achieves uniform charge and promotes uniform deposition of lithium ions through its own conductivity. The introduction of MXenes with unique accordion morphologies provides efficient charge transfer and lithium ion diffusion channels, accelerates the electrochemical reaction kinetics, induces the nucleation and growth of lithium ions on the surface of nanosheets, and effectively promotes the formation of polymer electrolyte- The lithium anode interface forms a uniform and dense interface layer, and the synergistic effect of AB and MXene improves the performance of the battery. The transport properties and charge distributions of AB and MXene were calculated using the DFTB+ module in Materials Studio. Lithium atoms are positively charged, while carbon atoms are negatively charged on the surface. The negative charge of MXene forms a charge band, which enables the uniform deposition of lithium ions and Effectively reduce the nucleation overpotential of lithium ions. More importantly, the MXene obtained after etching MAX with LiF and hydrochloric acid has a large number of −F ends, which can induce the formation of LiF (−Ti−F + Li+ + e−→−Ti + LiF) during cycling, and LiF is good. The high ionic conductivity and lower surface energy can facilitate reversible lithium ion transport and form a stable SEI film at the interface. After modification with AB and AB/MXene, respectively, the reversible capacity of the battery was greatly improved. The initial discharge specific capacities at 0.5 C cycle at room temperature are 111.1 mAh g-1 and 111.6 mAh g-1, respectively, and the discharge specific capacities after 100 cycles are 59.5 mAh g-1 and 91.2 mAh g-1, respectively. Even at a current density of 1 C, the battery with the AB/MXene modified layer can maintain a reversible capacity of 107.8 mAh g-1 with excellent cycling stability and rate performance.
The result was published online in the top international journal Energy & Environmental Materials (impact factor 13.443) with the title: A Charge Equalizer in Accordion—MXene-Modified Layer Leading to Spherical Lithium Deposition.
Zhao Yangmingyue is the first author of this article.
Graphical guide


Figure 2. SEM images of surfaces (a, c, e) and cross-sections (b, d, f) of unmodified PE (LOSP), AB-LOSP, and AB/MXene-LOSP PEs and SEM images of MXene (g). EDAX spectra of MXene (h) and AB/MXene-LOSP (i), tensile strength curves (j), combustion experiments of AB-LOSP (k) and AB/MXene-LOSP (l). These excellent physical properties will be the prerequisites for polymer electrolyte LIBs with good safety and electrochemical performance.

Figure 3. Ionic conductivity (a), effect of temperature on ionic conductivity (b), linear sweep voltammetry of LOSP, AB-LOSP, AB/MXene-LOSP PEs (c), AB/MXene-LOSP (d) ) and in situ EIS of AB-LOSP PEs (e). The SEI film induced by AB/MXene is beneficial to reduce the diffusion resistance of Li ions. The SEI film between the AB/MXene-LOSP electrolyte and the electrode is stable during battery cycling with good electrochemical performance.

Figure 4. EIS of symmetric lithium batteries composed of AB/MXene-LOSP PEs at different storage times (a), the effect of temperature on the interfacial impedance of lithium electrode-PEs (b), LOSP, AB-LOSP, AB/MXene-LOSP EIS bode diagram of the assembled LIBs (c), charge-discharge (d) and voltage-capacity (e) curves of LIBs assembled with LOSP, AB-LOSP, and AB/MXene-LOSP PEs at 0.5 C and room temperature, with different cycles The interfacial impedance of the number of times (f), the galvanostatic polarization curve of a symmetric battery with a current density of 0.05 mA cm-2 (g), the cycling curves at different rates (h), the capacity of the MCL interface modified LIB is comparable to the interface modification reported in the literature. Comparison of capacity retention of sexual LIBs (i). The MCL layer forms a more stable SEI film, which effectively reduces the interface impedance and polarization voltage, thereby improving the cycle performance and rate performance of the battery.

Summarize
In this paper, the electrolyte-anode interface modification layer is utilized to promote the uniform deposition of Li ions. The ionic conductivity of AB/MXene-LOSP PE reaches 5.21×10-4 S cm-1. The LiFePO4 battery assembled from AB/MXene-LOSP PE achieved a capacity of 91.2 mAh g-1 with a Coulombic efficiency of 99.83% after 100 cycles at 0.5 C and room temperature. Li nucleation experiments verify that the AB/MXene interfacial modification layer improves the uniform deposition and redox kinetics of Li. Because MXene and AB can tune the current density through uniform charge transfer. Therefore, lithium dendrites are suppressed, while spherical lithium is induced. In situ EIS investigated the beneficial effect of the modified layer on SEI film formation. SEM and XPS showed that the addition of etched MXene produced a LiF-enriched interfacial layer. A uniform SEI layer at the interface can reduce the overpotential (0.04 V) and achieve the cycling stability of the battery. This work will inspire our development in inducing uniform deposition of lithium ions and lowering the lithium ion transport barrier at the electrolyte-anode interface.
Literature link
https://doi.org/10.1002/eem2.12463
For the original text, please click the lower left corner of the tweet to read the original text
Special thanks to the author of this article for their strong support
- Previous: Coord. Chem. Rev.|Desi
- Next: MXene breakthrough: Na


 mxene academic
mxene academic