Summary: Scientific research results of Professor Chen Jun of Nankai in recent years
QQ Academic Group: 1092348845
Detailed
Chen Jun, an inorganic chemist, was born in Susong County, Anhui Province in September 1967. He joined the Communist Party of China in December 1988. He studied at the Department of Chemistry of Nankai University from 1985 to 1992. He obtained a bachelor‘s degree and a master‘s degree. He worked at the University of Wollongong University in Australia from 1996 to 1999, and obtained a doctorate degree. From 1999 to 2002, he worked as a researcher at the Japan New Energy and Industrial Technology Comprehensive Development Agency (NEDO) at the Japan Institute of Industrial Technology and Osaka Institute of Industrial Technology. Since 2002, he has been a professor and doctoral tutor of Nankai University. He was elected an academician of the Chinese Academy of Sciences in 2017. He is currently the dean of the School of Chemistry of Nankai University and the director of the Key Laboratory of Advanced Energy Materials Chemistry Ministry of Education.
In 2003, he was funded by the National Outstanding Youth Fund. In 2005, he was hired as a distinguished professor of the Yangtze River Scholars Award Program of the Ministry of Education. In 2010, he was employed as the 973 nanometer chief of the Ministry of Science and Technology. It plans to be a leader in scientific and technological innovation and won the China Electrochemical Contribution Award. In 2014, he was selected as a Fellow of the Royal Society of Chemistry (FRSC) and in 2016 was selected as the first batch of outstanding talents in Tianjin. Currently serves as the deputy editor of "Inorganic Chemistry Frontiers", "Science China Materials", "Applied Chemistry", "Solid State Sciences", "Nano Research", "ACS Eenergy Letters", "ACS Sustainable Chemistry & Engineering", "Journal of Energy" Editor of Chemistry, Journal of Chemistry, etc.
Mainly engaged in the research of inorganic solid chemistry. He has made important innovative contributions in the synthetic chemistry of inorganic solid functional materials, solid electrode preparation, and development and research of new battery electrode materials. A new "room temperature-redox-transformation" synthesis method was proposed. A stable conductive nano-spinel CoMn2O4 was synthesized at room temperature, which replaced the precious metal platinum electrode and was used in rechargeable metal lithium and zinc air batteries. The idea that the electrode micro-nanoization can improve the reactivity and structural stability of the multi-electron electrode is proposed. Micro-nano multi-level structure electrodes for rechargeable lithium, sodium, and magnesium batteries have been prepared through a large number of experiments, which improves the safety of the battery. The cost of electrode materials and the solution to the burning and explosion of batteries provide new ideas. Won the second prize of National Natural Science Award.
In order to facilitate everyone to quickly preview the scientific research results of Academician Chen Jun and his research team in recent years, the editors summarized the results propagated on the material source:
Science Advances: High-capacity aqueous zinc secondary battery with sustainable quinone electrode
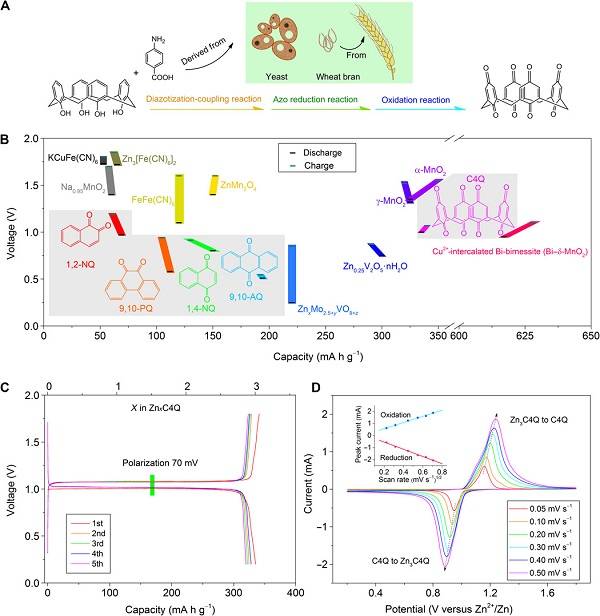
Academician Chen Jun‘s team published a research paper entitled "High-capacity aqueous zinc batteries using sustainable quinone electrodes" in Science Advances, and systematically studied the application of quinone electrodes in aqueous zinc batteries for the first time. Among them, researchers found that a cup [4] quinone (C4Q) positive electrode combined with a cation-selective membrane has a specific capacity as high as 335 mA h g-1 at a current density of 20 mA g−1 and an energy efficiency of 93%. Cycling 1,000 times at a current density of 500 mA g-1, the capacity retention rate reached 87%. At the same time, the soft-zinc-C4Q battery is based on the mass calculation of the C4Q positive electrode and the theoretical zinc negative electrode, and can provide an energy density of 220 Wh kg−1. The researchers also combined experimental research with theoretical calculations and developed an electrostatic potential calculation method to prove that carbonyl groups are electrochemically active centers. At the same time, the structural evolution and dissolution behavior of active materials during discharge and charging were studied by combining in-situ infrared, Raman, and ultraviolet-visible spectra. The mechanism of reversible electrochemical reaction was clarified. The article shows that batteries using quinone-based and metal anodes in water electrolytes are a promising large-scale energy storage option.
Literature link: High-capacity aqueous zinc batteries using sustainable quinone electrodes, (Science Adcances, 2018, DOI: 10.1126 / sciadv.aao1761)
Angew. Chem. Int. Ed .: Porphyrin-based Symmetric Redox Flow Battery for Energy Storage in Cold Climates
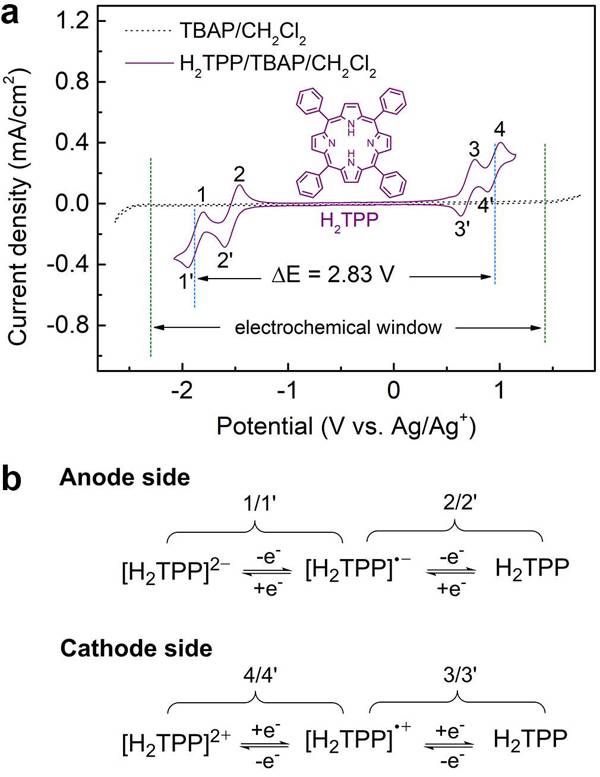
Under the leadership of Academician Chen Jun (corresponding author), a non-aqueous organic RFB based on 5,10,15,20-tetraphenylporphyrin (H2TPP) was constructed as a bipolar redox active material (anode: [H2TPP ] 2- / H2TPP, cathode: H2TPP / [H2TPP] 2+) and Y-type zeolite-polyvinylidene fluoride (Y-PVDF) ion-selective membrane with high ion conductivity as the separator. Porphyrin, as the active material of RFB, is derived from aromatic π-conjugated macrocycles with good electrical conductivity and reversible oxidation / reduction stability of molecular rings. The Y-PVDF ion-selective membrane separator retains H2TPP and redox products, and because of the pore exclusion effect, allows ClO4- rather than TBA + transport. The constructed RFB has a high capacity of 8.72 AhL-1 and a high voltage of 2.83 V in a temperature range of 20 ~ -40 ° C and excellent cycle stability (capacity retention rate per cycle exceeds 99.98%). Related results were published on Angew. Chem. Int. Ed. Under the title "Porphyrin-Based Symmetric Redox-Flow Batteries towards Cold-Climate Energy Storage".
Literature link: Porphyrin-Based Symmetric Redox-Flow Batteries towards Cold-Climate Energy Storage (Angew. Chem. Int. Ed., 2018, DOI: 10.1002 / ange.201713423)
Nature issue: high energy density, high power density rechargeable water-based zinc-manganese battery
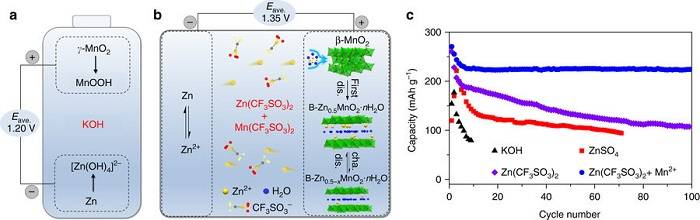
Professor Chen Jun, researcher Cheng Fangyi (co-corresponding author) and others published the latest research results "Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities" on Nature Communications. In this article, the researchers proposed the preparation of a high-performance rechargeable zinc-manganese dioxide battery using weakly acidic zinc trifluoromethanesulfonate / manganese salt as an electrolyte. The layered structure of the Zn-Buserite phase, the latter‘s layered structure enables reversible insertion and extraction of zinc ions. The manganese trifluoromethanesulfonate additive in the aqueous electrolyte inhibits the dissolution of Mn2 + and forms a uniform porous amorphous MnOx protective layer on the surface of the positive electrode, improving the positive electrode capacity and cycle stability of β-MnO2. In the end, the researchers assembled a soft-wrapped zinc-manganese battery with a total energy density of 75.2 Wh kg-1, which is much higher than other common water-based batteries. Due to its excellent performance, safety, simple process and cheap advantages, this rechargeable zinc-manganese battery system is expected to be applied to large-scale energy storage.
Literature link: Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities (Nat. Commun., 2017, DOI: 10.1038 / s41467-017-00467-x)
Nano Lett .: Antimony sulfide-graphene compound with high capacity and fast sodium ion storage performance
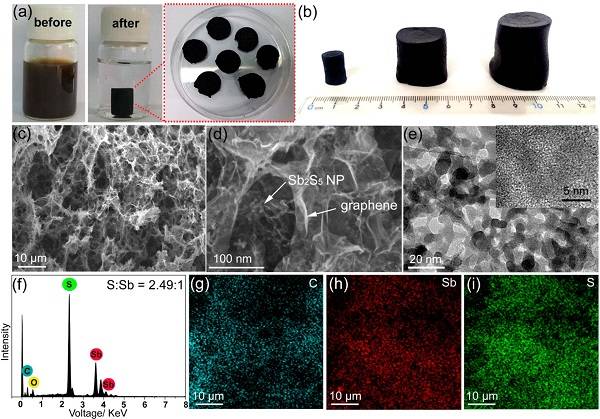
Professor Chen Jun and Lynden A. Archer of Cornell University (Communications) published a paper on Nano Lett. In this paper, a simple hydrothermal method was used to prepare Sb2S5 nanoparticles on graphene foam, and used it as a high-performance sodium electrode material. This material exhibits excellent performance, high capacity performance (at a current density of 0.1 A g−1, the capacity is 845 mA hg−1), and high cycle stability (cycles at a current density of 0.2 A g−1 for 300 cycles, capacity is maintained 91.6%), and good rate performance (current density 10.0 A g−1, capacity 525 mA hg−1). This new electrode material provides a new idea for the preparation of a safe, high energy density battery in the future.
Literature link: High-Capacity and Ultrafast Na-Ion Storage of a Self-Supported 3D Porous Antimony Persulfide–Graphene Foam Architecture (Nano Lett. 2017. DOI: 10.1021 / acs.nanolett.7b00889)
Angew. Chem. Int. Ed .: Flexible Li-CO2 Battery without Liquid Electrolyte

Professor Chen Jun (corresponding author) published an article entitled "Flexible Li-CO2 Batteries with Liquid-Free Electrolyte" on Angew. Chem. Int. Ed., Reporting a flexible Li-CO2 battery without liquid electrolyte. A polymethacrylate (PMA) / polyethylene glycol (PEG) -LiClO4-3 wt% SiO2 composite polymer electrolyte (CPE) and a multilayer carbon nanotube (CNTs) structured cathode were used. The battery has a stable structure and low interface resistance. After 100 charge and discharge cycles, its specific capacity remains at 1000 mAhg-1. In addition, the researchers performed electrochemical performance tests on assembled batteries with a size of 5 * 16 cm2. The batteries have excellent performance under different bending degrees (0-360o), with a charging and generating capacity of 993.3 mA · h and an energy density of up to 521 Wh˙kg-1, working time up to 220 h.
Literature link: Flexible Li-CO2 Batteries with Liquid-Free Electrolyte (Angew. Chem. Int. Ed., 2017, DOI: 10.1002 / anie.201701928)
Angew. Chem. Int. Ed .: Carbonate Materials for Fast-Charging Batteries
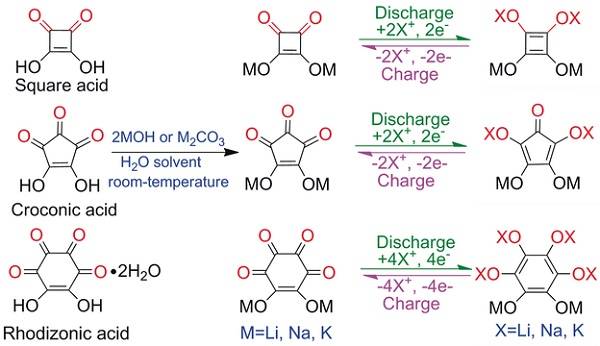
Professor Chen Jun (corresponding author) and others prepared organic oxometalates M (CO) n and studied their properties. The researchers used different metal ions and different structures of oxocarbons to find four-membered ring salts. It is more difficult to accept cations in a specific voltage region, and it is feasible for five-membered ring salts and six-membered ring salts to accept a certain number of cations. In addition, it was found that K2C6O6 (or K2C5O5) can be used as an ultra-fast insertion / deintercalation channel of potassium ions. DFT calculations show that K2C6O6 is a semiconductor with a narrow band gap close to 0.9eV. Potassium ion carbonate materials also have higher ionic conductivity than sodium ion and lithium ion carbonate materials, and the diffusion rate of potassium ions in the electrode material is also faster. The energy density of potassium ion batteries with K2C6O6 as the negative electrode and K4C6O6 as the positive electrode can reach 35 Wh · kg-1.
Literature link: Oxocarbon Salts for Fast Rechargeable Batteries (Angew. Chem. Int. Ed., 2016, DOI: 10.1002 / anie. 201607194)
Angewandte Chemie International Edition: New Cathode Materials and Multifunctional Binders

Professor Chen Jun‘s group has recently prepared a high-performance lithium-ion battery using dithioquinone (DTT) as the cathode and poly (3,4-ethylenedioxythiophene): poly (styrenesulfonate) (PEDOT: PSS) as the binder. Since there is a non-covalent bond interaction between DTT and PEDOT: PSS, this will help to suppress the dissolution of DTT and increase the conductivity of DTT, which will greatly improve the electrochemical performance. Using DTT as the cathode and PEDOT: PSS as the binder showed excellent cycle stability (292 mAh g-1 for the first cycle, 266 mAh g-1 after 200 cycles at 0.1 C) and high rate performance (at 1C 220 mAh g-1).
Original link: A Sulfur Heterocyclic Quinone Cathode and a Multifunctional Binder for a High-Performance Rechargeable Lithium-Ion Battery
Adv. Energy Mater .: Phosphide of selenium (Se4P4) is used as anode material of sodium ion battery
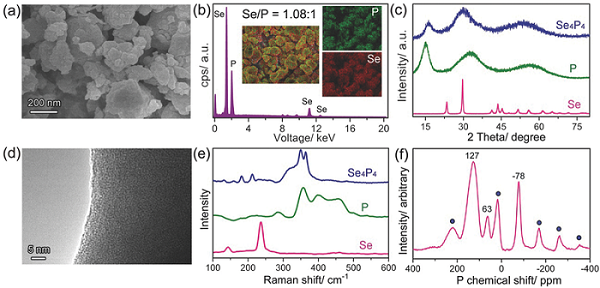
The research team of Professor Chen Jun (corresponding author) published an article titled Selenium Phosphide (Se4P4) as a New and Promising Anode Material for Sodium-Ion Batteries on Advanced Energy Materials. Selenium (Se) is an interesting material in the field of sodium ion batteries. It has high electrical conductivity and theoretical capacity. In addition, Se has better cycle stability than Sn. Pure Se will form easily soluble intermediate products during the reaction process, resulting in rapid decay of capacity; and combining Se with P elements will effectively avoid the appearance of intermediate products. In this study, a simple mechanical grinding method was used to prepare an amorphous Se4P4 material, and it proved that it can be used as a negative electrode material for sodium ion batteries. The experimental results show that Se4P4 reacts with sodium ions to directly generate Na2Se during cycling, has a reversible capacity of 1048 mA h g-1 and high cycle stability under 50 mA g-1 charge and discharge conditions ( The capacity is 804 mA h g-1). In addition, under 500 mA g-1 charge and discharge conditions, its reversible capacity is 724 mA h g-1, and the capacity of 332 mA h g-1 is retained even under 3000 mA g-1. Compared with previously reported phosphides, Se4P4 has a higher reversible capacity, better rate performance and cycle stability.
Original link: Selenium Phosphide (Se4P4) as a New and Promising Anode Material for Sodium-Ion Batteries. (Adv. Energy Mater., 2016, DOI: 10.1002 / aenm.201601973)
Adv. Energy Mater: New Additives Promote Large-area Printing of Organic Polymer Solar Cells
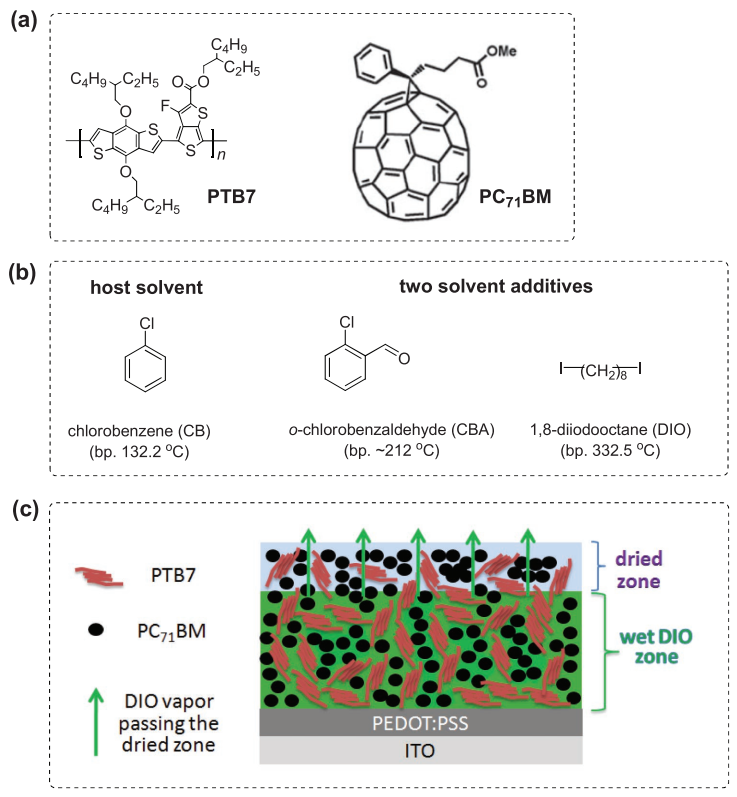
Professor Chen Junwu (corresponding author), Zhang Lianjie (corresponding author), academician Cao Yu and professor Liu Yi (corresponding author) from Shanghai Jiaotong University jointly synthesized an additive o-chlorobenzaldehyde (CBA, o-chlorobenzaldehyde). Compared with the conventional additive 1,8-diiodooctane (DIO, diiodooctane), the boiling point of CBA is reduced from 332.5 ° C to 212 ° C, which is more easily removed when the active layer is vacuum dried, thereby affecting its morphological characteristics. When this additive is applied in the classic PTB7: PC71BM system, when the thickness of the active layer is 200-300nm, the PCE efficiency of the active layer using CBA is 9.11% and 8% compared to the active layer using DIO as an additive.
Reference link: Using o-Chlorobenzaldehyde as a Fast Removable Solvent Additive during Spin-Coating PTB7-Based Active Layers: High Effciency Thick-Film Polymer Solar Cells (Adv. Energy Mater., 2016, DOI: 10.1002 / aenm.201601344)
Adv. Funct. Mater. Sea-urchin-like structure CoSe2 used as anode material for high-performance sodium ion batteries
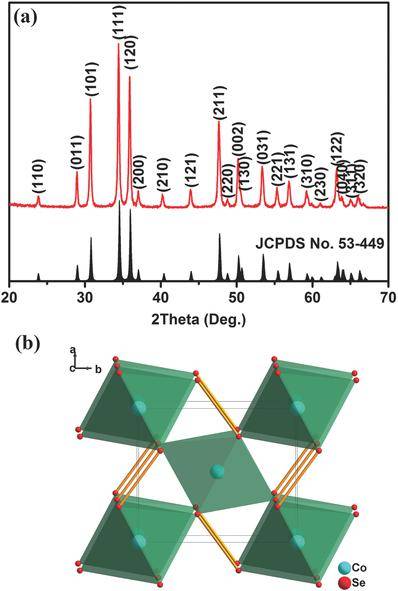
Studies have found that by optimizing the electrolyte and voltage window, transition metal chalcogenides can achieve high rate performance and long-term cycling. Among them, CoSe2 has a structure similar to pyrite, which can be widely used in hydrogen evolution, Li-O2 batteries, water oxidation catalysts, and electrodes of dye-sensitized solar cells and supercapacitors. When CoSe2 is used as a negative electrode material for lithium ion batteries, it shows good cycle performance and rate performance, but research on sodium ion batteries has been carried out less. The research team of Professor Chen Jun from the Key Laboratory of Energy Materials Chemistry of the Ministry of Education of Nankai University and the research team of Professor Yong-Mook Kang of Dongguk University in South Korea jointly studied the performance of CoSe2 as a negative electrode material for sodium ion batteries.
Literature link: Urchin-Like CoSe2 as a High-Performance Anode Material for Sodium-Ion Batteries
Joule Review: Solid Sodium Electrolyte and Its Interface Engineering
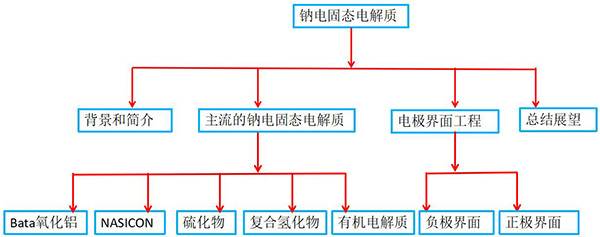
Academician Chen Jun‘s team wrote a review article entitled "Electrolyte and Interface Engineering for Solid-State Sodium Batteries" on Joule. This article systematically summarizes the development and latest progress of sodium ion solid electrolyte and the internal interface problems of solid sodium batteries. The basic principles of solid electrolyte design with high ionic conductivity and high chemical / electrochemical stability are discussed. Aiming at the key issues in the development of high-performance solid-state sodium batteries, the interface design between the electrolyte and the electrode was emphasized.
Literature link: "Electrolyte and Interface Engineering for Solid-State Sodium Batteries" (DOI.org/10.1016/j.joule.2018.07.028)
AM review: Application of molecular engineering optimized carbonyl electrode materials in solid / liquid rechargeable batteries
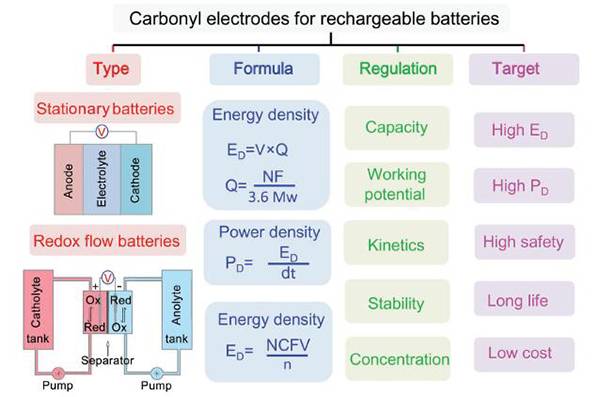
Professor Chen Jun (corresponding author) ‘s research group recently published a review entitled "Molecular Engineering with Organic Carbonyl Electrode Materials for Advanced Stationary and Redox Flow Rechargeable Batteries" on Advanced Materials, summarizing the use of molecular engineering methods to effectively regulate carbonyl The capacity, working voltage, active material concentration, kinetics, and stability of solid-state / flow batteries, and solve some problems of organic carbon electrode materials. This review shows the basic principles and some creative ideas for the application of carbonyl electrode materials in electrode materials for next-generation secondary batteries.
Literature link: Molecular Engineering with Organic Carbonyl Electrode Materials for Advanced Stationary and Redox Flow Rechargeable Batteries (Adv. Mater., 2017, DOI: 10.1002 / adma.201607007)
Source of information: material cattle
- Previous: [ICF] Synthesis of Rh
- Next: Bioactive Materials |


 Academic Frontier
Academic Frontier
