Nano enzymes, different style / latest results of nano enzymes delivery
QQ Academic Group: 1092348845
Detailed
1
Nano Letters Ferritin Nanozymes Protect Blood-Brain Barrier Integrity Against Malaria
Cerebral malaria is a fatal complication caused by malaria infection that is characterized by central nervous system dysfunction, and usually cannot be effectively treated by combined antimalarial therapies. Among them, the isolation of the interaction between cerebrovascular endothelial cells and parasite-infected red blood cells and the destruction of the blood-brain barrier play a key role in the pathogenesis. Academician Yan Xiyun of the Institute of Biophysics of the Chinese Academy of Sciences has developed a ferritin nanozyme composed of recombinant human ferritin, which targets the blood-brain barrier endothelial cells and the catalase-like Fe3O4 nanozyme Used to remove active oxygen from the body. When administered to mice with cerebral malaria, ferritin nanozymes promote the elimination of malaria in the blood by protecting the blood-brain barrier endothelial cells from damage by reactive oxygen species and by polarizing macrophages to type M1, Reduces parasitemia, which significantly improves survival. The ferritin nanozyme significantly reduced brain inflammation and memory disorders in cerebral malaria mice treated with artemether. The combined use of ferritin nanozyme and antimalarial drugs provides a new strategy for the treatment of cerebral malaria. [1] Related research was published in Nano Letters under the title "Fenozyme Protects the Integrity of the Blood-Brain Barrier against Experimental Cerebral Malaria".
https://pubs.acs.org/doi/abs/10.1021/acs.nanolett.9b03774
Figure 1: Schematic diagram of ferritin nanozymes protecting mice from experimental brain malaria

2
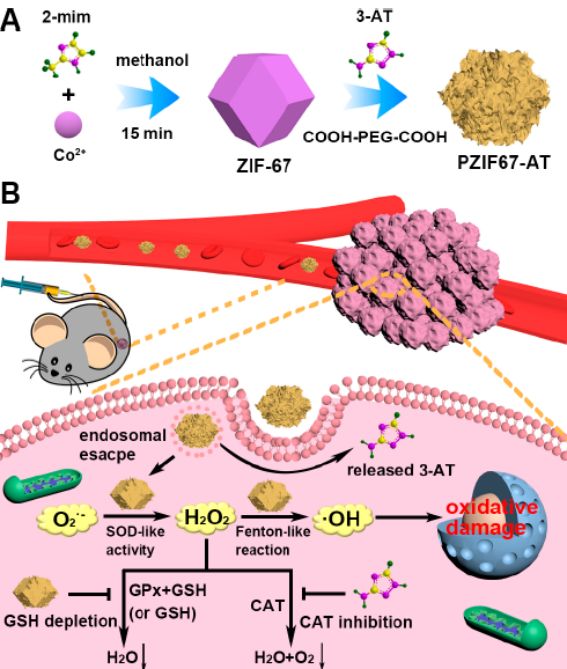
Angew. Chem. Int. Ed. Monoatomic Nanozyme Antibacterial
Monoatomic catalysts have been widely used in chemical catalysis, but whether monoatomic nanozymes have good catalytic activity has important research significance. The research group of Academician Yan Xiyun of the Institute of Biophysics of the Chinese Academy of Sciences and the research group of Professor Liu Huiyu of Beijing University of Chemical Technology have proposed a zinc-based zeolite-imidazolium skeleton (ZIF-8) derived carbon atom material containing dispersed zinc atoms. Monoatomic peroxidase mimics. Experiments show that the single-atom nanozyme has similar catalytic activity and M-Nx active site as the natural enzyme, and the high catalytic activity is attributed to the coordination unsaturated Zn-N4 active site. The single-atom nanozyme promotes the decomposition of hydrogen peroxide and the formation of hydroxyl radicals, and has an inhibition rate of 99.87% on Pseudomonas aeruginosa, and significantly promotes wound healing. In a wound model of in vivo infection, the single-atom nanozyme can significantly promote wound healing without significant toxicity to various tissues and organs. This work opens up new horizons for monoatomic catalysts in the field of enzyme catalysis and biological applications. [2] A related study, entitled "Single-Atom Nanozyme for Wound Antibacterial Applications", was published in Angew. Chem. Int. Ed.
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201813994
Figure 2: Schematic of single-atom nanozyme for wound antibacterial
3
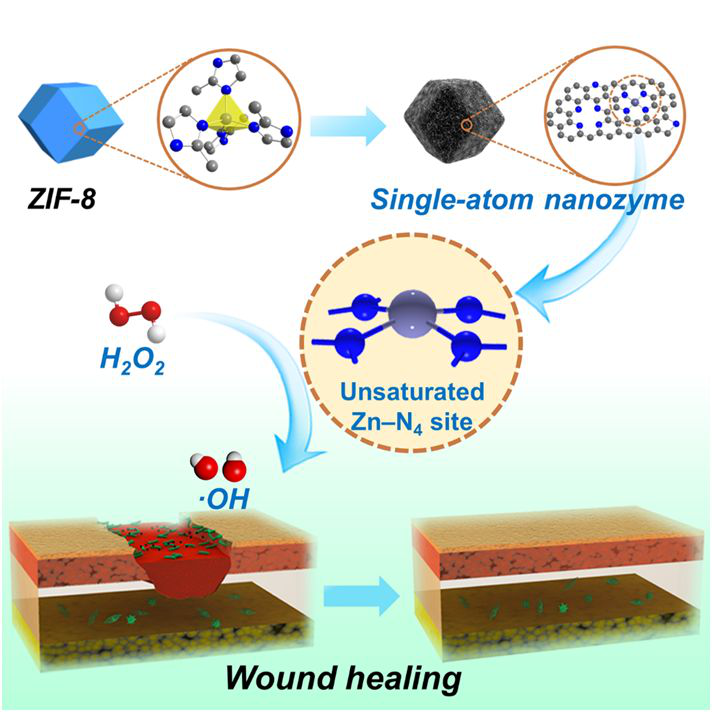
Nano Letters Hydrogen Peroxide-Responsive Nano Enzyme for Photoacoustic Imaging of Nasopharyngeal Carcinoma
Due to its advantages in deep tissue penetration and fine spatial resolution, photoacoustic imaging is expected to be used in clinical cancer diagnosis. Academician Yan Xiyun‘s research group of Institute of Biophysics, Chinese Academy of Sciences and Nie Guohui‘s research group of Shenzhen Second People‘s Hospital have proposed an exosomes-like nano-enzyme vesicle with hydrogen peroxide response for photoacoustic imaging of nasopharyngeal carcinoma. Graphene quantum dot nanozyme (GQDzyme) with peroxidase-like activity can diammonium 2, 2‘-diazepine bis (3-ethylbenzothiazoline-6-sulfonic acid) in the presence of hydrogen peroxide Salt (ABTS) is effectively converted to its oxidized form (oxABTS), where oxABTS has a strong near-infrared absorption rate, making it an ideal contrast agent for photoacoustic imaging. GQDzyme / ABTS nanoparticles were biomimetically functionalized with folic acid-modified natural red blood cell membranes to construct exosomal-like nanozyme vesicles for photoacoustic imaging of nasopharyngeal carcinoma. The exosome-like nanozyme vesicles can effectively accumulate in nasopharyngeal carcinoma tissues and selectively trigger catalytic photoacoustic imaging in nasopharyngeal carcinoma tissues. Compared with normal tissues, due to the rapid metabolism of the tumor, the nanoenzyme vesicle can sensitively detect the relative increase of hydrogen peroxide in nasopharyngeal carcinoma, and has excellent stealth ability, prolongs the circulation time, and improves the tumor Accumulation. This work is the first application of a nanoenzyme-based catalytic imaging strategy in vivo, providing a new idea for the treatment of nasopharyngeal carcinoma. [3] Related research was published in Nano Letters under the title "Exosome-like Nanozyme Vesicles for H2O2‑Responsive Catalytic Photoacoustic Imaging of Xenograft Nasopharyngeal Carcinoma".
https://pubs.acs.org/doi/abs/10.1021/acs.nanolett.8b03709
Figure 3: Photoacoustic Imaging of Catalytic Nanozyme Vesicles for Nasopharyngeal Carcinoma Tumors
Xiaogang Qu (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences)
4
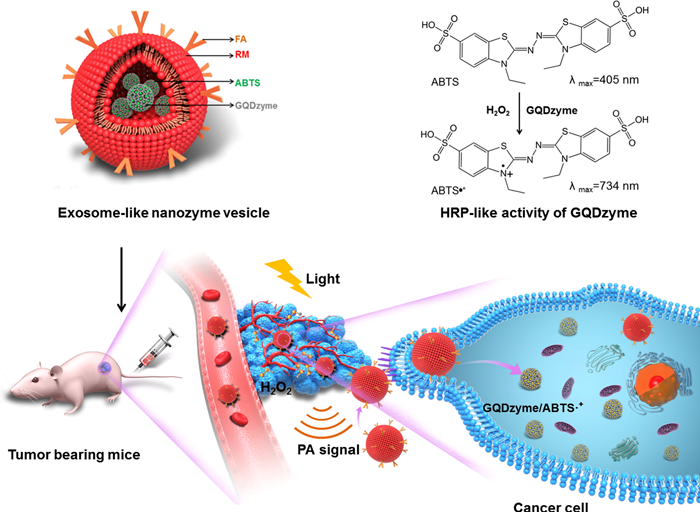
ACS Nano Enzymes as Self-Activating Cascade Agents for Wound Healing in Vivo
Since the optimal reaction of peroxidase occurs in a strongly acidic environment with a pH value of 3-4, the application of metal organic framework (MOF) with peroxidase-like activity at neutral pH in the biomedical field is severely limited Rarely, and the direct introduction of a relatively high concentration and toxic reaction reagent H2O2 will cause certain damage to normal tissues. Professor Qu Xiaogang of the Changchun Institute of Applied Chemistry of the Chinese Academy of Sciences has used the ultra-thin two-dimensional MOF nanosheets as peroxidase simulants to prepare a MOF nanozyme complex by physically adsorbing glucose oxidase. The complex acts as a benign and self-activating cascade reagent for antibacterial in vivo and in vitro. Glucose oxidase can continuously convert non-toxic glucose into rich gluconic acid and H2O2, avoiding the direct use of relatively high concentrations and toxic H2O2, and reducing the pH value of the entire system to 3-4, minimizing side effects . By catalyzing glucose to peroxidase-like activity of self-activated 2D MOF nanosheets, a large amount of H2O2 produced at the same time can be used for subsequent 2D MOF nanosheets to catalyze, thereby increasing the production efficiency and antibacterial capacity of highly toxic and OH. This design of benign and self-activating cascade reagents has powerful antibacterial effects, negligible biological toxicity, and will promote the application of MOF-based nanozymes in the fields of biochemistry and biomedicine. [4] Related research was published in ACS Nano under the title "Two-Dimensional Metal-Organic Framework / Enzyme Hybrid Nanocatalyst as a Benign and Self-Activated Cascade Reagent for in Vivo Wound Healing".
https://pubs.acs.org/doi/abs/10.1021/acsnano.8b09501
Figure 4: Schematic of preparation and antibacterial activity of MOF nanozyme complex
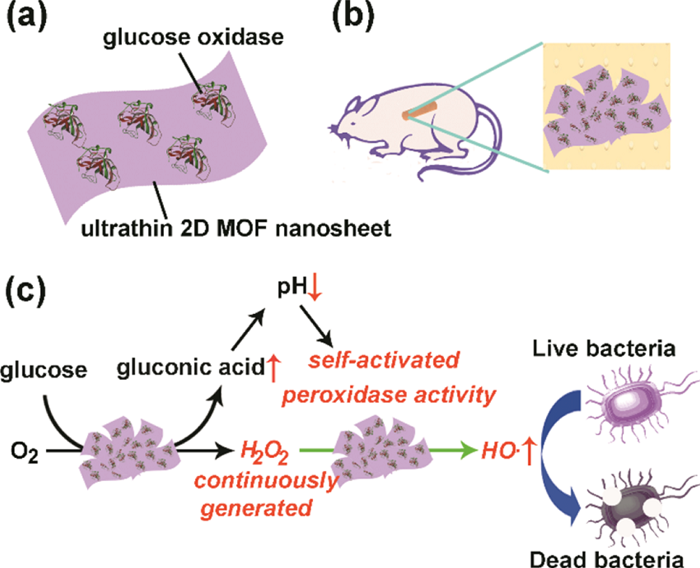
5
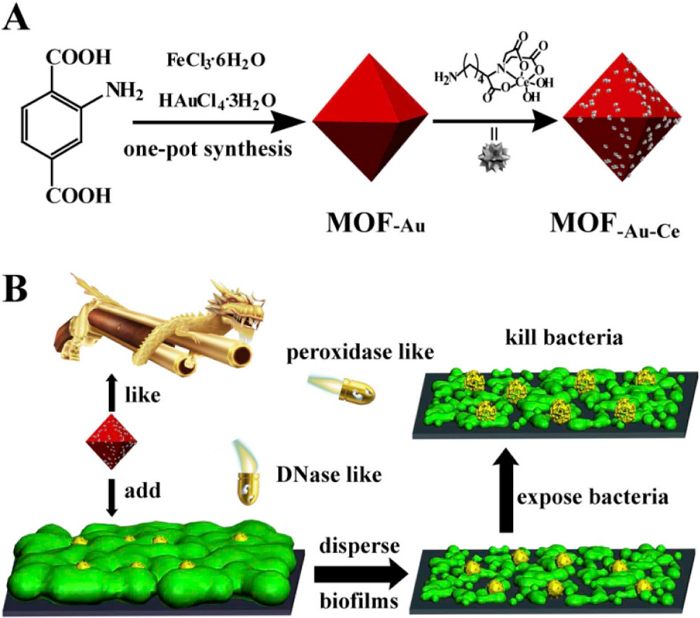
Biomaterials nanozymes disrupt biofilms and hinder bacterial proliferation
Bacterial biofilms are posing a serious threat to global public health. Extracellular DNA, as an important component of extracellular polymers in biofilms, can connect different extracellular polymer components with bacteria, making it difficult to eliminate biofilms. Professor Qu Xiaogang‘s group from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences has designed and synthesized a series of MOF / Ce nanozymes with peroxidase mimicking activity to combat biofilms. Cerium (IV) complex with deoxyribonuclease activity can hydrolyze extracellular DNA and destroy established biofilms, while MOF with peroxidase-like activity can kill bacteria dispersed in biofilms in the presence of H2O2, This will prevent recolonization of the bacteria and recurrence of the biofilm. The combination of two nanoenzymes is a reasonable strategy to obtain enhanced anti-biofilm properties. This nanoenzyme with dual enzyme mimicking activity can penetrate biofilms and strongly inhibit the formation of bacterial biofilms. This work is helpful for the design of collaborative multi-nanozyme systems and provides new ideas for more in-depth applications of MOF nanozymes. [5] Related research was published in Biomaterials under the title "A series of MOF / Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria".
https://www.sciencedirect.com/science/article/pii/S0142961219302182
Figure 5: Schematic diagram of MOF / Ce nanozymes for anti-biofilm applications
6
Angew. Chem. Int. Ed. Defective Nanozyme Enhances Bacterial Inhibition as a New Antibiotic
Nanozymes are a new generation of antibiotics with broad-spectrum antibacterial properties and low biological toxicity. However, nanozymes cannot capture bacteria and have low catalytic activity, resulting in unsatisfactory antibacterial effects. Professor Qu Xiaogang‘s group from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences has proposed nanoenzymes with rough surfaces and defect-rich active edges for antibacterial research. Due to the lower adsorption energy of H2O2 and the desorption energy of OH * and the larger exothermic process of the entire reaction, compared with the original nanoenzyme, the rough surface increases the adhesion of bacteria, and the defect-rich edges show more High peroxidase-like activity. In addition, the introduction of congenital photothermal effects leads to higher therapeutic effects both in vitro and in vivo. This work not only developed a new strategy to produce high-efficiency nanozymes with bacterial capture capabilities as alternative antibiotics, but also provided new insights into the design of biomaterials by integrating nanotopology, defect incorporation, and catalytic properties. [6] A related study entitled "Defect-Rich Adhesive Nanozymes as Efficient Antibiotics for Enhanced Bacterial Inhibition" was published in Angew. Chem. Int. Ed.
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201908289

7
JACS Nanozyme H2O2 homeostasis destroyer for enhanced chemodynamic therapy
Chemodynamic therapy (CDT) has gained widespread attention by activating tumor cells by endogenous stimulation of tumors. CDT utilizes Fe2 + -mediated Fenton reaction or Cu2 + or Mn2 + -mediated Fenton reaction in tumor cells to convert less reactive hydrogen peroxide to hydroxyl radicals, and the reactive oxygen species (ROS) produced can induce cell decay. Death and necrosis, thereby killing tumor cells. Professor Qu Xiaogang‘s group at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences has proposed a method to increase the hydrogen peroxide content in tumor cells by using nano enzymes. Nanozymes can promote the production of hydrogen peroxide and inhibit the decomposition of hydrogen peroxide to increase the hydrogen peroxide content in tumor cells, which can be used to enhance the killing effect of CDT on tumor cells. Experiments have shown that nanoenzyme complexes can promote the synthesis of hydrogen peroxide in cancer cells while inhibiting the consumption of hydrogen peroxide by glutathione and decomposition by catalase, effectively increasing the level of hydrogen peroxide in tumor cells. Hydrogen peroxide is converted into hydroxyl free radicals that have a killing effect on tumor cells. The development of this method provides a new idea for other activation methods to improve the treatment capacity of CDT. [7] A related study, entitled "Bioinspired Construction of a Nanozyme-Based H2O2 Homeostasis Disruptor for Intensive Chemodynamic Therapy", was published in J. Am. Chem. Soc.
https://pubs.acs.org/doi/abs/10.1021/jacs.9b12873
Figure 7: Schematic of nanoenzyme complex synthesis and chemodynamic therapy

8
Adv. Funct. Mater. Nanoenzyme hydrogel enhances ability to capture and destroy bacteria
The widespread multidrug resistance caused by the abuse of antibiotics has prompted researchers to explore new ways to treat bacterial infections. Nanozymes can mimic the functions of natural enzymes to induce the production of highly toxic reactive oxygen species (ROS) as antibacterial agents. However, the lack of effective interactions between nanoenzymes and bacteria, and the inherent short life span and diffusion distance of intrinsic reactive oxygen species have greatly reduced their bactericidal activity. Professor Qu Xiaogang‘s group at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences has developed a positively charged and porous MoS2 nanoenzyme-hydrogel to achieve enhanced antibacterial agents. Nanoenzyme-hydrogels with positively charged and macroporous properties can trap and limit bacteria within the scope of ROS destruction. By combining the near-infrared photothermal properties of the MoS2 nanoenzyme, the nanoenzyme-hydrogel can achieve a synergistic bactericidal effect, eliminate bacteria and reduce the risk of inflammation, and skin wound healing will accelerate. The original strategy of MoS2 nanoenzyme-hydrogel to improve the antibacterial performance of nanoenzyme and promote wound healing. The combination of nanoenzyme and hydrogel reduces the damage of MoS2 nanoenzyme to tissues, provides better options for effective elimination of bacteria and better biological safety, and can provide new insights into the broad antibacterial research of nanoenzymes. [8] The related research was titled "Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria" and was published in Adv. Funct. Mater.
https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.201900518
Figure 8: Preparation of MoS2 nanoenzyme-hydrogel and its treatment for removing bacteria and wound infections

Wei Hui (Nanjing University)
9
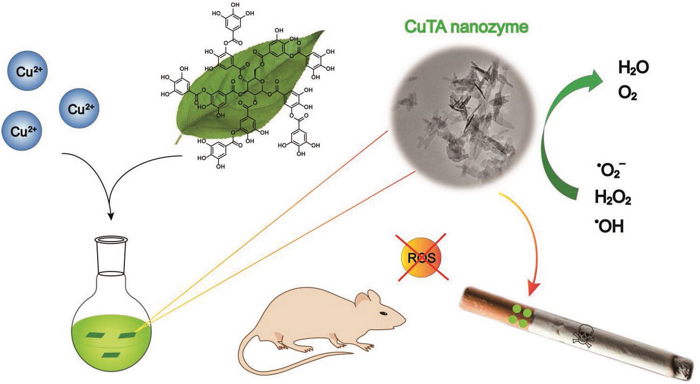
Small Nanozyme used to remove ROS from cigarette smoke
Global tobacco continues to be a devastating threat to public health. Toxic reactive oxygen species (ROS) in cigarette smoke cannot be effectively eliminated by currently available cigarette filters, and the resulting oxidative stress can cause severe lung injury and other diseases. Professor Wei Hui‘s group at Nanjing University proposed the use of a new copper tannic acid coordination (CuTA) nanoenzyme as a highly active and thermally stable ROS scavenger. CuTA nanozyme exhibits superoxide dismutase-like activity, catalase-like activity and hydroxyl radical elimination ability. These synergistic antioxidant capabilities make CuTA nanozymes a potential application area for improving commercial cigarette filters. Commercial cigarettes loaded with CuTA nano enzymes can effectively remove ROS from cigarette smoke, reduce lung inflammation caused by oxidative stress, and minimize acute lung injury. CuTA nanozymes provide effective ROS scavengers with multiple antioxidant capabilities, opening new opportunities for modifying cigarette filters to reduce the toxicity of cigarette smoke. [9] Related research was published in Small. The topic was "Copper Tannic Acid Coordination Nanosheet: A Potent Nanozyme for Scavenging ROS from Cigarette Smoke".
https://onlinelibrary.wiley.com/doi/full/10.1002/smll.201902123
10
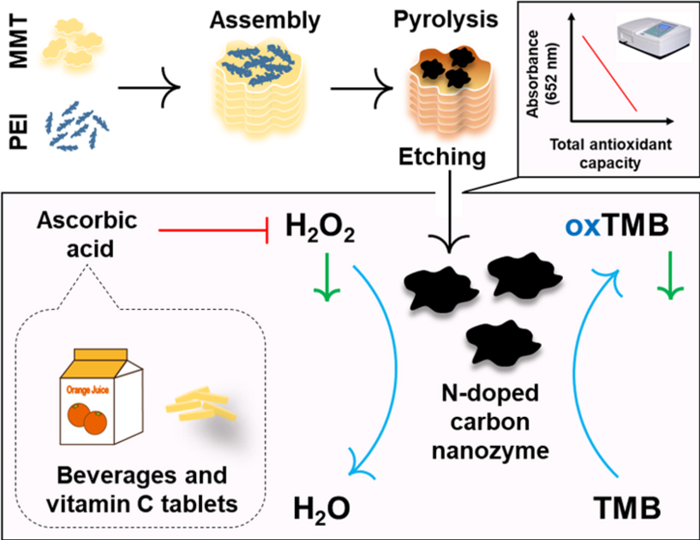
Anal. Chem. Nanozyme for determination of total antioxidant capacity
Since the restructured electronic structure in a nitrogen-doped carbon nanozyme is beneficial for catalytic processes, a nitrogen-doped strategy is used to enhance the activity of the carbon nanozyme. However, it is still difficult to obtain a carbon nanozyme having a high nitrogen content due to the instability of the nitrogen element at a high calcination temperature. Professor Wei Hui‘s group at Nanjing University proposed a new nitrogen doping strategy, using a highly nitrogen-containing polymer (polyethyleneimine (PEI)) as a nitrogen source and a natural clay mineral (montmorillonite (MMT)) as a template To prepare a highly active peroxidase-like carbon nanozyme. The assembly of MMT with PEI protects the loss of nitrogen elements at high calcination temperatures, thereby retaining more catalytically active nitrogen sites. Hydroxyl radicals are key intermediates for peroxidase-like catalysis. H2O2, glucose, and ascorbic acid were detected by using highly active and specific carbon nanozymes, and total antioxidant capacity was tested in four commercial beverages. This study not only provides a new strategy for preparing peroxidase-like nanozymes, but also develops a simple method for measuring total antioxidant capacity that can be used in the future to evaluate the quality of antioxidant foods and oxidative stress in healthcare . [10] A related study, titled "N-Doped Carbon As Peroxidase-Like Nanozymes for Total Antioxidant Capacity Assay", was published in Anal. Chem.
https://pubs.acs.org/doi/pdf/10.1021/acs.analchem.9b04333
Figure 10: Preparation of carbon nanozymes and determination of their total antioxidant capacity
11
Anal. Chem. Fluorescent Nanozyme Ratio Biosensor
Most current nanoenzyme biosensor systems are based on a single signal output. This detection system is easily affected by environmental and personal factors, but nanoenzyme sensing systems with proportional signal output will provide more reliable and powerful sensing. performance. Professor Wei Hui‘s group at Nanjing University prepared three g-C3N4 fluorescent nanozymes with peroxidase-like activity, and based on the g-C3N4-Ru nanozyme, constructed a hydrogen peroxide and its related metabolites Proportional fluorescence sensor. Fluorescent nanozyme emits 438 nm fluorescence when excited at 385 nm. In the presence of H2O2 and nanozyme, o-phenylenediamine is catalytically oxidized to oxOPD. Among them, oxOPD can not only emit fluorescence at 564 nm, but also quench the nanozyme. Fluorescence at 438 nm. The ratio of the fluorescence intensity at 564 nm and 438 nm was used as the signal output to construct a proportional biosensor system for the detection of phosphate and hydrogen peroxide and their related metabolites. This study proposes a new method for constructing a proportional nanozyme biosensor system. [11] Related research was published in Anal. Chem. Under the title "Fluorescent Graphitic Carbon Nitride-Based Nanozymes with Peroxidase-Like Activities for Ratiometric Biosensing".
https://pubs.acs.org/doi/abs/10.1021/acs.analchem.9b01884
Figure 11: Schematic biosensing of g-C3N4 fluorescent nanozyme ratio

12
Nature Communications eg occupancy as an effective assessment of the catalytic activity of perovskite nanoenzymes
Peroxidase is used to catalyze the oxidation of peroxide substrates. However, there is no predictive description. The exploration of peroxidase and other enzyme nanomaterials (called nanozymes) mainly relies on trial and error strategy. Professor Wei Hui‘s group at Nanjing University studied the occupancy of eg orbitals as an indicator for evaluating the peroxidase-like activity of transition metal oxides (including perovskite oxides). Since oxygen plays a central role in these bionic catalytic reactions, eg orbital occupancy may control the peroxidase-like activity of perovskite-type transition metal oxides. ABO3 type perovskite transition metal oxide with BO6 octahedral subunit was used as a model system to study the relationship between its catalytic activity and eg orbital occupancy, and to adjust the orbital occupancy regulation by adjusting the ABO3 composition. Both experimental tests and density functional theory calculations revealed the volcanic relationship between the occupancy of eg orbitals and nanoenzyme activity, of which the peroxidase-like activity was the highest, corresponding to an occupancy rate of about 1.2 for orbitals. This study provides an in-depth understanding of the catalytic mechanism of nanozyme peroxidase-like activity and the relationship between the adaptive structure and catalytic activity of nanozymes, and provides further exploration of the occupancy of eg orbitals to predict the enzyme-like activity of other metal oxides. Got new insights. [12] A related study, titled "eg occupancy as an effective descriptor for the catalytic activity of perovskite oxide-based peroxidase mimics", was published in Nature Communications.
https://www.nature.com/articles/s41467-019-08657-5
Figure 12: Schematic study of perovskite transition metal oxide peroxidase activity

references
1. Zhao S., Duan H., Yang Y., et al. Fenozyme Protects the Integrity of the Blood-Brain Barrier against Experimental Cerebral Malaria. [J] Nano Lett. 2019, 19, 8887-8895.
2. Xu B., Wang H., Wang W., et al. Single-Atom Nanozyme for Wound Antibacterial Applications. [J] Angew. Chem. Int. Ed. 2019, 58, 4911-4916.
3. Ding H., Cai Y, GaoL., Et al. Exosome-like Nanozyme Vesicles for H2O2‑Responsive Catalytic Photoacoustic Imaging of Xenograft Nasopharyngeal Carcinoma. [J] Nano Lett. 2019, 19, 203-209.
4. Liu X., Yan Z., Zhang Y., et al. Two-Dimensional Metal-Organic Framework / Enzyme Hybrid Nanocatalyst as a Benign and Self-Activated Cascade Reagent for in VivoWound Healing. [J] ACS Nano 2019, 13, 5222-5230.
5. LiuZ., WangF., Ren J., et al. A series of MOF / Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. [J] Biomaterials 2019, 208, 21-31.
6. Cao F., Zhang L., Wang H., et al. Defect-Rich Adhesive Nanozymes as Efficient Antibiotics for Enhanced Bacterial Inhibition. [J] Angew. Chem. Int. Ed. 2019, 58, 16236-16242.
7. SangY., Cao F., Li W., et al. Bioinspired Construction of a Nanozyme-Based H2O2 Homeostasis Disruptor for Intensive Chemodynamic Therapy. [J] J. Am. Chem. Soc. DOI: 10.1021 / jacs.9b12873.
8. Sang Y., Li W., Liu H., et al. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. [J] Adv. Funct. Mater. 2019, 29, 1900518.
9. Lin S., Cheng Y., Zhang H., et al. Copper Tannic Acid Coordination Nanosheet: A PotentNanozyme for Scavenging ROS from Cigarette Smoke. [J] Small 2019, 1902123.
10. Lou Z., Zhao S., Wang Q., et al. N-Doped Carbon As Peroxidase-Like Nanozymes for Total Antioxidant Capacity Assay. [J] Anal. Chem. 2019, 91, 15267-15274.
11. Wang X., Qin L., Lin M., et al. Fluorescent Graphitic Carbon Nitride-Based Nanozymes with Peroxidase-like Activities for Ratiometric Biosensing. [J] Anal. Chem. 2019, 91, 10648-10656.
12. Wang X., Gao X., Qin L., et al. Egoccupancy as an effective descriptor for the catalytic activity of perovskite oxide-based peroxidase mimics. [J] Nature Communications 2019, 10, 704.
Source of information: material source
- Previous: [Fourth COF] 3D PAF fi
- Next: IF 55.8! Toward Effici


 Academic Frontier
Academic Frontier