CEJ: Thermal reduction of sulfur-containing MAX phase
QQ Academic Group: 1092348845
Detailed
【Research Background】
Two-dimensional transition metal carbides, carbonitrides and nitrides (MXenes) are the main class of materials in the emerging 2D nanomaterial family. In some energy-related applications, such as rechargeable batteries and supercapacitors attention. This is mainly due to its outstanding conductivity and ability to accommodate various ions between adjacent layers. Up to now, some harmful etching agents, such as fluorine-containing acid solutions, have been widely used in the synthesis of MXene. This method may cause serious security risks, unpredictable environmental pollution problems and insufficient reliability of large-scale industrial production. At the same time, the selective etching method has strict requirements on the acid concentration and etching time, so it is difficult to achieve a uniform MXene sample. In addition, the use of fluoric acid will form a passive layer on the active edge or surface of MXenes. Previous density functional theory predicted that fluorine-containing functional groups on the surface will affect the transport of lithium ions and reduce the electrochemical lithium storage capacity.
【Achievement Introduction】
Recently, Professor Sun Ziqi of Queensland University of Technology in Australia and Professor Hu Chunfeng of Southwest Jiaotong University published research papers titled: Thermal reduction of sulfur-containing MAX phase for MXene production in the internationally renowned academic journal Chemical Engineering Journal . The paper reports an innovative thermal reduction strategy to synthesize MXene obtained from the corresponding sulfur-containing MAX as a precursor, where the weakly bonded S atoms react with hydrogen to form a volatile gas, thereby obtaining Ti 2 C in 2D graphene Nanosheets.
【Graphic introduction】
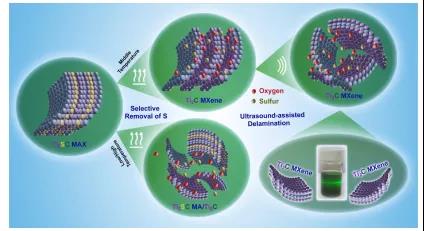
Figure 1. Schematic diagramof2D Ti 2 C MXeneobtained by efficient thermal reduction method.

Figure 2. The XRD image of 2D Ti 2 SCMAX under different temperature conditions , the temperature range is 600 ~ 900 ℃; MXene film after peeling treated with acetone.
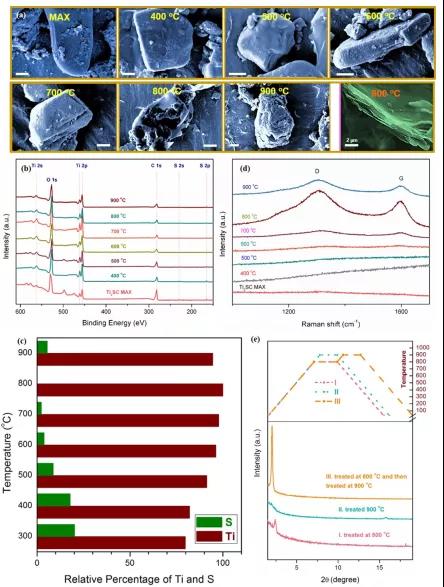
Figure 3. Relevant physical characterization of 2D Ti 2 SC MAX phase under different temperature conditions.

Figure 4. SEM image of 2D Ti 2 SC MAX, element mapping and TEM image of 2D Ti 2 C MXene.
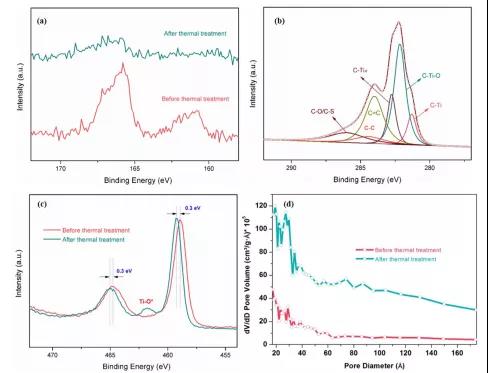
Figure 5. XPS pattern and pore size distribution of 2D Ti 2 SC MAX and 2D Ti 2 C MXene.

Figure 6. 2D Ti 2 C MXene lithium storage electrochemical performance test.

Figure 7. 2D Ti 2 C MXene torsion loss diagram before and after cycling and TEM and HRTEM images after cycling.
【Summary of this article】
This paper proposes an innovative thermal reduction sulfur-containing MAX phase strategy to synthesize MXene. The study found that the optimal reduction temperature was 800 ° C, and the obtained MXene exhibited a unique 2D sheet structure. As an anode material for lithium ion batteries, the prepared MXene has an initial discharge capacity of ~ 200 mAh g -1 . Even at a higher current density (2,000 mA g -1 ), the capacity can be maintained at a relatively stable level of ~ 70 mAh g -1 after 130 cycles , with good rate performance and cycle stability. This work opened a new door for the preparation of large-scale MXene and promoted the application of MXene-based materials in the field of rechargeable batteries.
Literature link:
https://doi.org/10.1016/j.cej.2020.125111
Source: MXene Frontier
- Previous: Catalysis B: MXene ass
- Next: Advanced Materials | A


 mxene academic
mxene academic
