ACSAEM: MXene-based
QQ Academic Group: 1092348845
Detailed
¡¾Research Background¡¿
In recent years, the excessive use of fossil fuels has caused prominent problems (energy crisis). At the same time, the problems related to the energy crisis have become increasingly serious, prompting the rapid development of new energy development and utilization. Therefore, the development of efficient energy storage devices has become the focus of global attention. Considering the cycle performance and power density, the performance of super capacitors (SC) is superior to many other electrochemical energy storage devices, which has attracted worldwide attention. Among the widely studied electrode materials for SC, Ti 3 C 2 T x (MXene) has excellent physical and chemical properties, making it a promising electrode material for SC.
¡¾Achievement Introduction¡¿
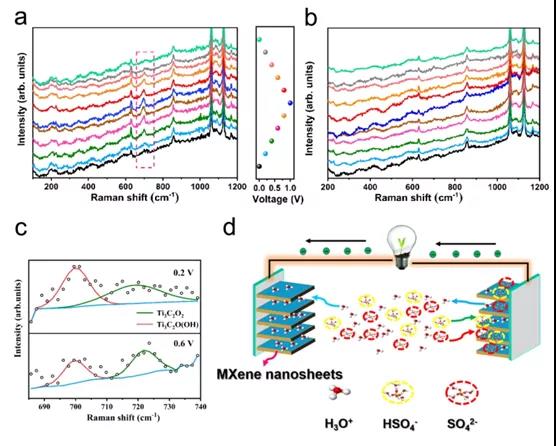
Recently, Professor Wenxiu Que, Professor Ya-Ping Du of Xi¡¯an Jiaotong University and Professor Lingbing Kong of Shenzhen University of Technology published an article in the internationally renowned academic journal ACS Applied Energy Materials : Understanding MXene Based ¡°Symmetric¡± Supercapacitors and Redox Electrolyte Energy Storage The research paper of this study investigated the symmetrical SC based on Ti 3 C 2 T x by in-situ Raman spectroscopy and X-ray diffraction . The results show that the bonding / debonding of the redox of Ti-O and hydrogen ions occurs at On the negative electrode, the positive electrode mainly involves pseudocapacitors embedded with hydronium ions. At the same time, the voltage between the two electrodes in the H 2 SO 4 electrolyte is distributed asymmetrically. Due to the partial oxidation of the positive electrode, the negative electrode shows a much larger capacitance than the positive electrode. Therefore, a strategy by introducing a redox active substance (KI) into a traditional H 2 SO 4 electrolyte is proposed . The results show that converting the positive electrode into a battery-type capacitor expands the working potential range of the negative electrode, thereby significantly increasing the capacitance of the SC. In addition, the energy density of the SC based on Ti 3 C 2 T x in the mixed electrolyte is 33.2 Wh L-1 , much higher than the energy density of H 2 SO 4 electrolyte (14.3 WhL -1 ). This method can be used as an efficient strategy to improve the energy storage capacity of SC based on MXene.
¡¾Graphic introduction¡¿
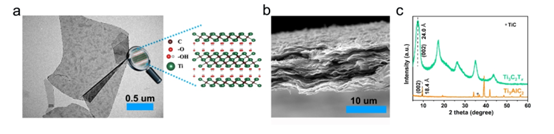
FIG. 1. (A) of Ti . 3 C 2 T X the TEM image and a schematic view of the crystal structure of nano-sheet (B) of Ti . 3 C 2 T X cross-sectional SEM image of (c) film of Ti . 3 C 2 T X film and of Ti . 3 XRD pattern of AlC 2 powder
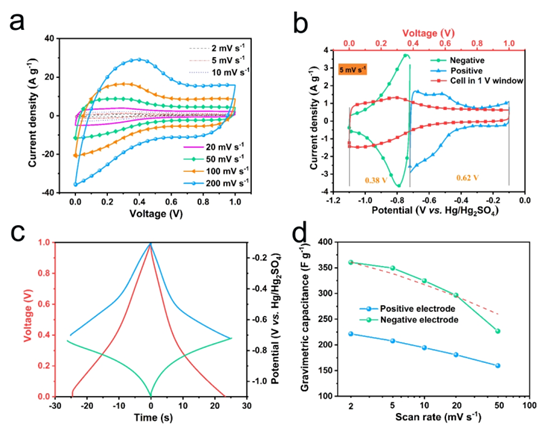
Figure 2. (a) CV curve of symmetric SC based on MXene. (B) CV curve and (c) GCD curve of positive and negative electrodes (d) mass specific capacitance
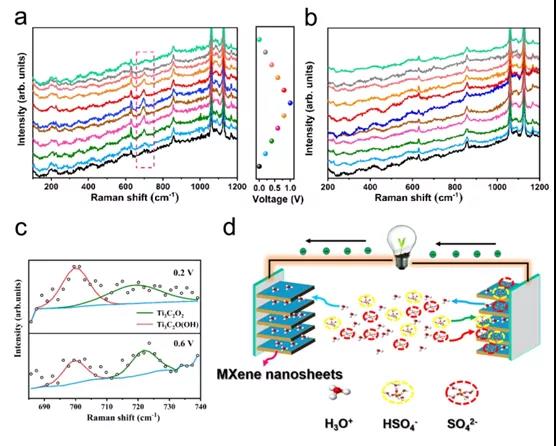
Figure 3. In-situ electrochemical Raman spectroscopy of negative electrode (a) and positive electrode (b) (c) Selected spectrum of positive electrode and Lorentz fitting band (d) Schematic diagram of working mechanism of symmetric SC
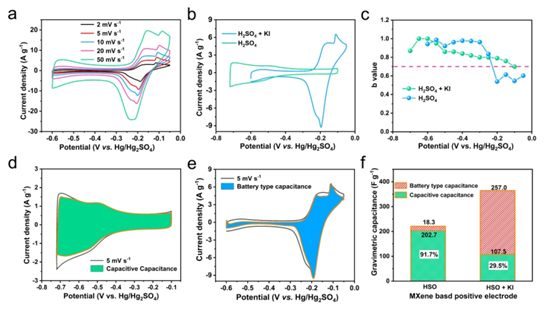
Figure 4. (a) CV curve of MXene electrode in mixed electrolyte (b) CV curve of MXene electrode in different electrolytes (c) b value of MXene electrode in different electrolytes (d) capacitive capacitance pair in H 2 SO 4 electrolyte Contribution of total capacitance (e) Contribution of battery-type capacitors in mixed electrolytes to total capacitance (f) Contribution of positive electrodes in different electrolytes to total mass capacitance
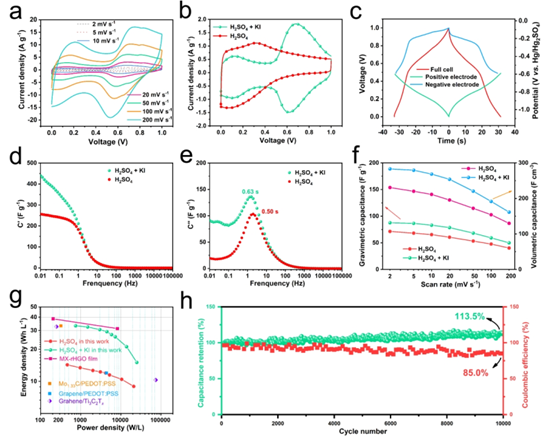
Figure 5. (a) CV curve of SC in mixed electrolyte (b) CV curve of SC in different electrolytes (c) GCD curve of SC, positive electrode and negative electrode in mixed electrolyte. H 2 SO . 4 mixed electrolyte SC actual capacitance (d) and imaginary capacitance (e) with the variation of the frequency (f) at different scan rates of mass capacitance and volumetric capacitance (G) H 2 SO . 4 and mixed electrolyte Ragone diagram of SC (h) Cycling performance of Ti 3 C 2 T x based SC at 10 A g -1 in mixed electrolyte
¡¾Summary of this article¡¿
This paper confirms that the ¡°symmetric¡± SC based on Ti 3 C 2 T x thin film exhibits asymmetrical voltage splitting between the negative electrode and the positive electrode in H 2 SO 4 electrolyte, where the energy storage performance of SC is affected by the lower positive electrode Limitation of energy storage capacity. The electrochemical reaction of the negative electrode involves the redox of Ti-O, accompanied by the bonding / debonding of hydronium ions, and the adsorption / desorption of HSO 4 - and SO 4 2- also exists in the intercalation of hydrogen ions in the intercalation. By adding a redox active substance (ie KI) to the traditional H 2 SO 4 electrolyte, the capacitance of the positive electrode can be increased, thereby converting the positive electrode into a battery type, which can greatly increase the energy density of the SC. In addition, the improvement in the energy storage capacity of the positive electrode can be attributed to the insertion of I 3- / I - into MXene. The volumetric energy density of the SC is as high as 33.2Wh L -1 , which is significantly better than the volumetric energy density of the original SC. Due to the increased interlayer spacing, SC can remain 11.35% after 10,000 cycles in the mixed electrolyte. Therefore, this method can be used as a reference for MXene-based storage systems with high energy density.
Literature link:
https://doi.org/10.1021/acsaem.0c00527
Source:
- Previous£º AEM: Femtosecond laser
- Next£º Advanced Materials | A


 mxene academic
mxene academic