The recent achievements of the team of Professor Wang Chunsheng from the University of Maryland
QQ Academic Group: 1092348845
Detailed
1. Nature Energy: LiF-rich solid-electrolyte interface can be used for high-performance battery ultrafine alloy negative electrode
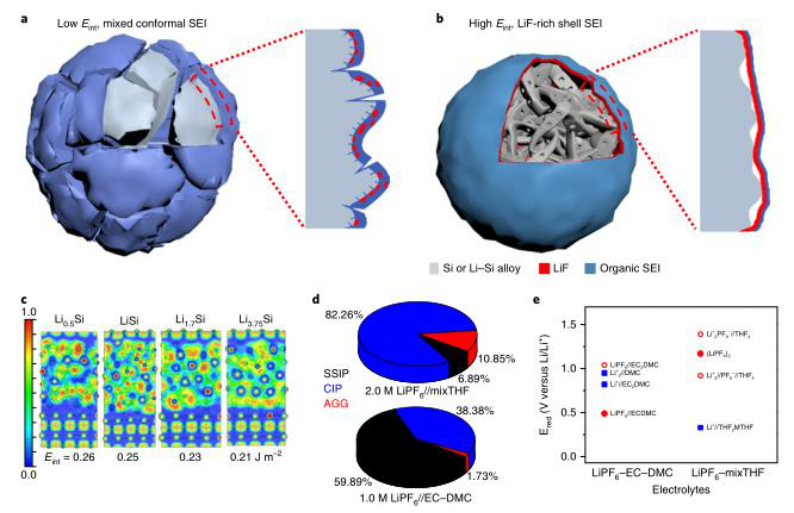
Lithium batteries with Si, Al or Bi micron (more than 10 ¦Ìm) particulate anodes have the advantages of high capacity, easy production, low cost and low environmental impact, but they also have the disadvantages of rapid degradation and low Coulomb efficiency. In view of this, Professor Wang Chunsheng of the University of Maryland and Oleg Borodin of the U.S. Army Research Laboratory reported that through a properly designed electrolyte (2.0 M LiPF 6 in a 1: 1 v / v mixture of tetrahydrofuran and 2-methyltetrahydrofuran ) 100 The cycle of a full-filled battery is composed of ultra-fine Si, Al and Bi anodes with commercial LiFePO 4 and LiNi 0.8 and Co 0.15 Al 0.05 O cathodes.
Article main points:
1) The inner layer of SEI in contact with the alloy material is a pure inorganic material, and the interface energy with the lithiated alloy is high, and the mechanical strength is high, so as to adapt to the large volume change of the anode of the alloy. At the same time, the electrolyte can selectively form such LiF-organic double-layer SEI on the micro-alloy negative electrode, so that SiMPs, AlMPs and BiMPs undergo elastic and plastic deformation in the SEI shell.
2) SiMPs in the electrolyte have high capacities of 2800 mAh g -1 and 5.6 mAh cm -2 in more than 400 cycles, with an initial CE of 91% and a cycle CE exceeding 99.9%. The SiMP // LFP full battery has a high cycle CE close to 100% and a cycle life of more than 100 cycles at an actual capacity greater than 2.0 mAh cm -2 . AlMP // LFP, BiMP // LFP and SiMP // NCA full-cell initial cycles also showed stable and excellent performance.
3) By forming a high-modulus LiF-organic double-layer interface, the electrode material performance can be promoted, in which LiF and the alloy negative electrode have a high interface energy to adapt to the plastic deformation of the lithium alloy during cycling.
This work provides a simple and practical solution for current battery technology without any modification of adhesives or special manufacturing methods.
Chen, J., Fan, X., Li, Q. et al. Electrolyte design for LiF-rich solid¨Celectrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat Energy (2020).
DOI: 10.1038 / s41560-020-0601-1
https://doi.org/10.1038/s41560-020-0601-1
2. JACS: structure and interface design to achieve a stable lithium-rich positive electrode
The layered lithium-rich oxide positive electrode has the highest theoretical energy density among all inserted positive electrodes and can be used in high-energy lithium-ion batteries. However, due to the low initial coulombic efficiency (CE) of the layered oxide cathode of the O3 structure, continuous release of oxygen causes severe voltage decay and poor cyclic stability, as well as structural rearrangement and delithiation caused by irreversible transition metal migration A serious side reaction between the positive electrode and the electrolyte. Therefore, its practical application is severely limited.
In view of this, in order to solve the above problems, Professor Wang Chunsheng of the University of Maryland and Enyuan Hu of Brookhaven National Laboratory reported the stable O2 structure of Li 1.2 Ni 0.13 Co 0.13 Mn 0.54 O 2 ( O 2 -LR-NCM) and perfluorinated»¯ electrolyte.
Article main points:
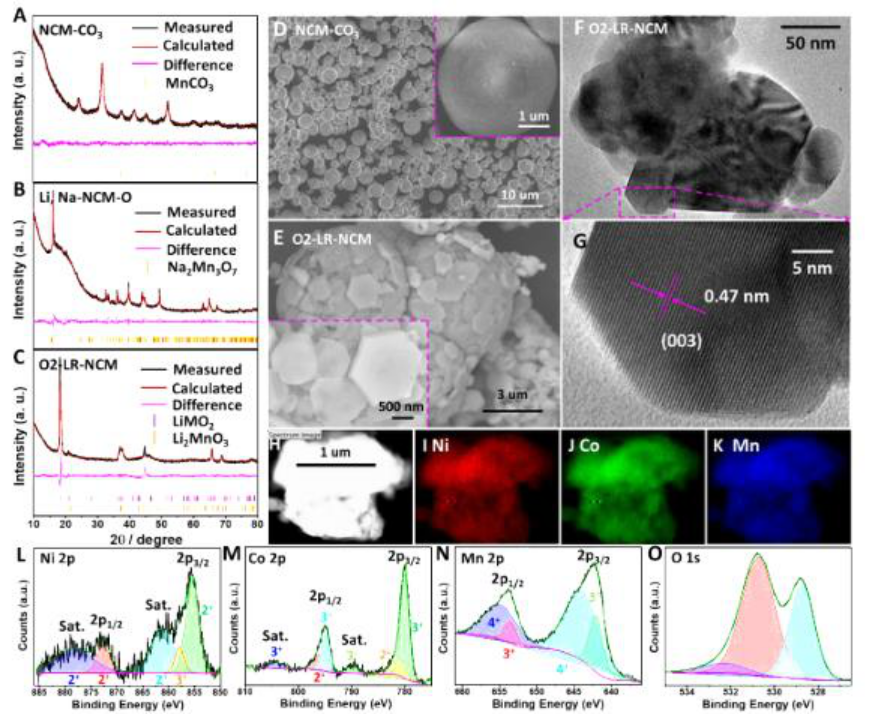
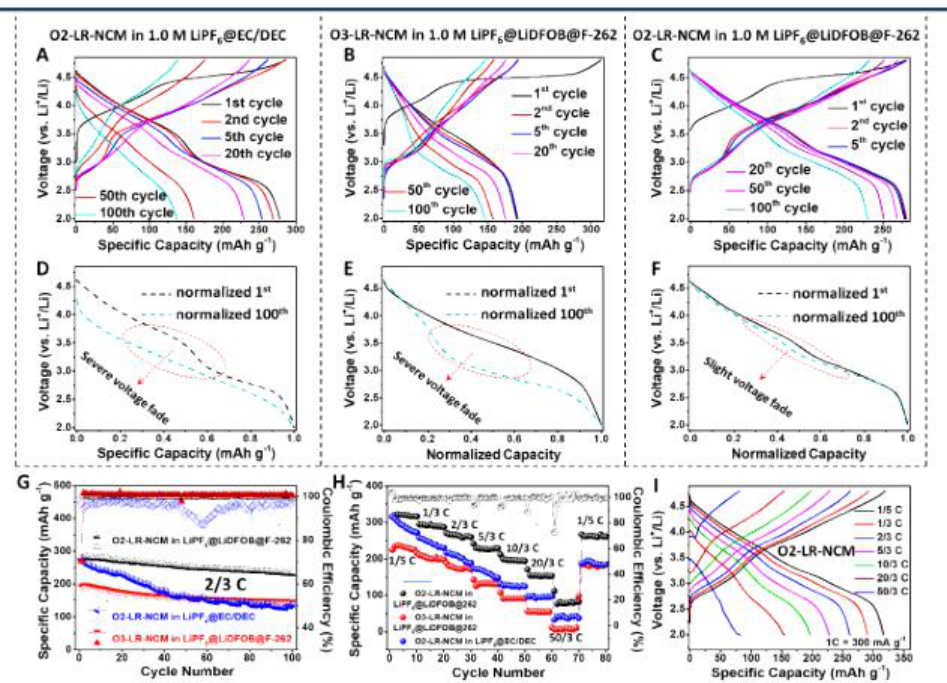
1) researchers first, prepared by co-precipitation reaction of Ni 0.13 of Co 0.13 Mn 0.54 (CO.¡®S . 3 ) 0.8 ( Step NCM-CO3), after which the-CO.¡®S the NCM . 3 and of Na 2 CO.¡®S . 3 and of Li 2 CO.¡®S . 3 of The mixture was annealed to prepare Na 5/6 Li 1/4 (Ni0.13Co0.13Mn0.54) 3/4 O x ( Li, Na-NCM-O), which can be indexed as P2 type Na 2 Mn 3 O 7 structure. Then, Li, Na-NCM-O was immersed in a molten salt mixture of LiNO 3 / LiCl (88:12, w / w) to perform an ion exchange process, and the resulting product was O2-LR-NCM.
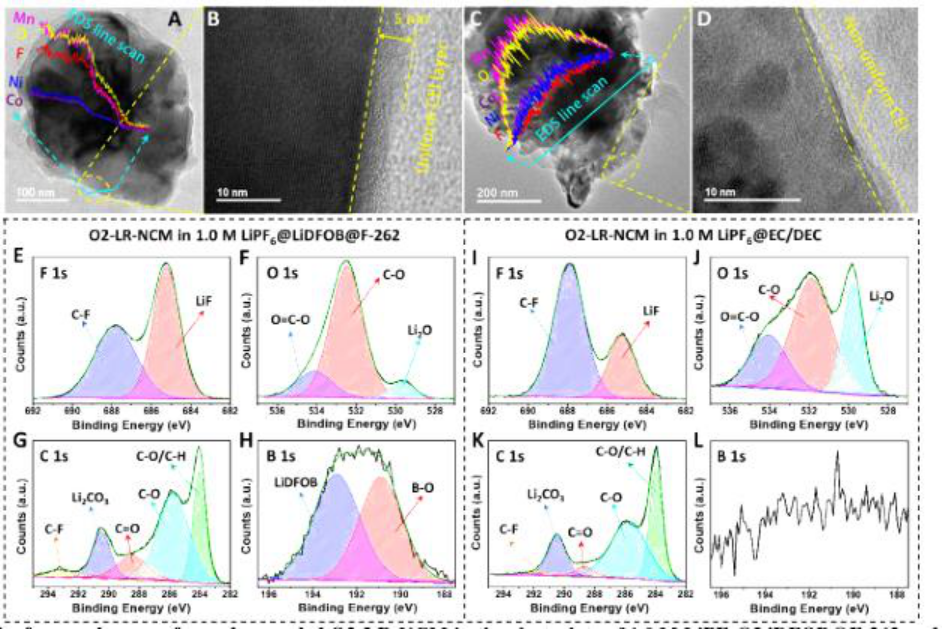
2) O2-LR-NCM can effectively limit the transition metal migration to the Li layer and the fluorinated cathode electrolyte mesophase (CEI) formed in situ on the surface of O2-LR-NCM, preventing it from fully fluorinated electrolyte in the initial cycle break down. Inhibit structural transformation and oxygen evolution, thereby protecting transition metal redox pairs to achieve a highly reversible and stable redox reaction.
2) O2-LR-NCM in all fluorinated electrolytes can achieve a high initial CE of 99.82%, a cycle CE exceeding 99.9%, a high reversible capacity of 278 mAh / g and a high capacity retention rate after 100 cycles (83.3% ).
The study shows that the collaborative design of the electrolyte and cathode structure is an effective way to stabilize the high energy cathode.
Chunyu Cui, et al, Structure and Interface Design Enable Stable Li-Rich Cathode, J. Am. Chem. Soc., 2020
DOI: 10.1021 / jacs.0c02302
https://doi.org/10.1021/jacs.0c02302
3. JACS: high interface energy interface helps safe lithium metal batteries
Constructing a stable solid electrolyte interface (SEI) is very important to inhibit the growth of metallic lithium dendrites. However, due to the complexity of the electrochemical reaction, it is difficult to form a specific SEI film by adjusting the electrolyte composition. Recently, Fudong Han of American Cyril University of Technology, Professor Wang Chunsheng of University of Maryland, and Professor Tu Jiangping of Zhejiang University have got rid of the traditional trial and error method of constructing SEI films by using traditional electrolyte components, using Li- with 11% Li content The Sr alloy anode constructed a stable solid electrolyte interface rich in SrF 2 in a perfluorinated electrolyte .
The main points of this article:
1) Using theoretical calculations and experimental characterization, the researchers found that the SrF 2 rich solid electrolyte interface has a higher interface energy and greater mechanical strength for lithium metal, so it can promote the lateral growth and improvement of the deposited lithium metal by The stability of the SEI film suppresses dendrite growth.
2) in 2M LiFSI-DME in the electrolyte, Li-Sr // Cu half-cell current density of 1mA / cm2 and 1 mAh of / cm & lt 2 coulombic efficiency up to volume 99.42%, in 3mA / cm & lt 2 current density and of 2mAh / cm & lt 2 coulombic efficiency at high capacities 98.95%. Li-Sr // Li-Sr symmetrical batteries can also maintain a stable metal lithium deposition -stripping cycle for more than 180 h at an ultra-high current density of 30 mA / cm 2 and a capacity of 1 mAh / cm 2 .
3) The researchers matched the composite negative electrode with LiFePO 4 positive electrode and LiNi 0.8 Co 0.1 Mn 0.1 O 2 positive electrode. The electrochemical performance of the whole battery in different electrolytes was also very stable. This work has opened the way for the construction of specific SEI films for regulating the composition of metal lithium anodes .
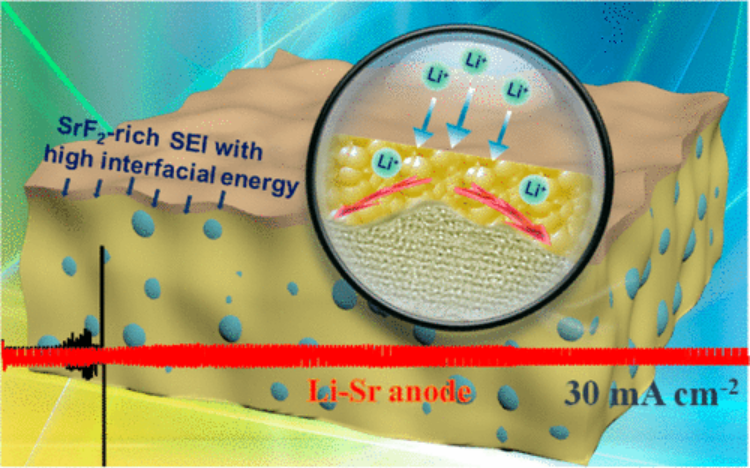
Sufu Liu, Fudong Han, Jiangping Tu, Chunsheng Wang et al, High Interfacial-Energy Interphase Promoting Safe Lithium Metal Batteries, JACS, 2020
Doi: 10.1021 / jacs.9b11750
https://pubs.acs.org/doi/abs/10.1021/jacs.9b11750
4. Nano Energy: Redox reaction of cations and anions in P2 layered sodium ion oxide
The demand for high specific energy sodium ion batteries has promoted the in-depth study of the high energy oxygen redox chemical reaction process in layered transition metal oxide cathodes. However, most layered cathodes with oxygen reduction reactions may produce irreversible electrochemical reactions, resulting in rapid capacity decay and potential O 2 release. Recently, Professor Wang Chunsheng of the University of Maryland and others reported that the highly electronegative copper element can stabilize the sodium-deficient P2-Na 2/3 Mn 0.72 Cu 0.22 Mg 0.06 O 2 phase, thereby realizing cation and anion redox chemistry.
The main points of this article:
1) Hard X-ray and soft X-ray absorption spectra show that all Mn 3+ / Mn 4+ , Cu 2+ / Cu 3+ and O 2- / (O2) n- participate in oxidation after sodium ion extraction and insertion Reduction reaction. Density functional theory ( DFT) calculations confirm that the strong covalentity between copper and oxygen ensures the redox activity of cations and anions in the P2-Na 2/3 Mn 0.72 Cu 0.22 Mg 0.06 O 2 phase.
2) P2-Na 2/3 Mn 0.72 Cu 0.22 Mg 0.06 O 2 positive electrode shows a stable cycle life, the capacity retention rate of 100 cycles at 1C is 87.9%, and high rate performance (at 10C cycle is 70.3 mA hg -1 ).
3) Their findings not only provide guidelines for improving the electrochemical performance of layered oxides based on anion redox activity, but also explore the underlying scientific principles behind oxygen redox processes.
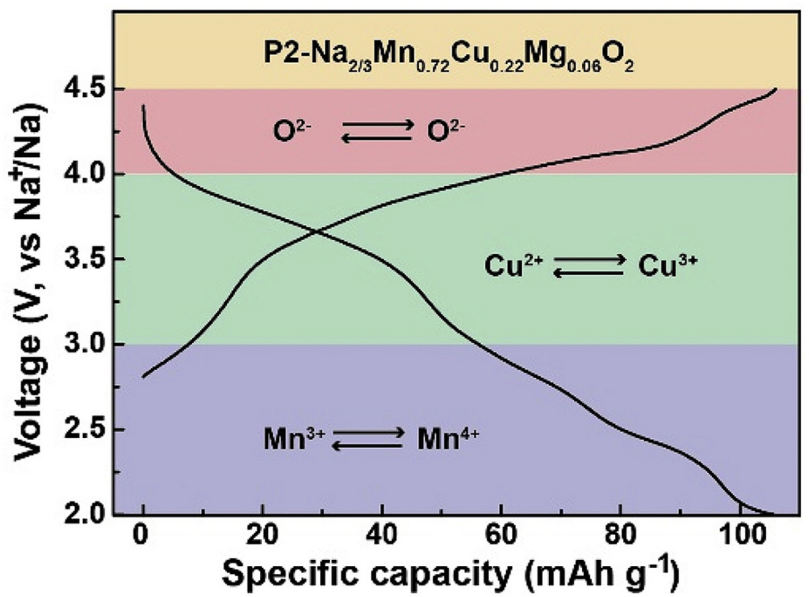
Peng-FeiWang, YaoXiao, NanPiao, Qin-ChaoWang, XiaoJi, TingJin, Yu-JieGuo, SufuLiu, TaoDeng, ChunyuCui, LongChen, Yu-GuoGuo, Xiao-QingYang, Chunsheng Wang, Both Cationic and Anionic Redox Chemistry in a P2-Type Sodium Layered Oxide, Nano Energy 2020
DOI: 10.1016 / j.nanoen.2020.104474
https://doi.org/10.1016/j.nanoen.2020.104474
5. AM: Adjust the chemistry of the anode-electrolyte interface of the garnet-based solid-state lithium battery
Lithium metal (Li) is a very promising anode material for high energy density solid state batteries. However, interface problems, including large interface resistance and the generation of lithium dendrites, have always hampered the commercialization of solid-state lithium metal batteries (SSLB). In view of this, Professor Wang Chunsheng of the University of Maryland and Professor Zhang Jiguang of the Pacific Northwest National Laboratory reported a new type of LPO @ LLZTO composite material, which solved the most challenging interface problem between the lithium metal anode and the garnet-type LLZTO solid electrolyte.
Article main points:
1) A thin layer of solid electrolyte Li 3 PO 4 (LPO) is coated on the surface of GSE by atomic layer deposition (ALD) and a simple annealing process . The LLZTO pellets with ultra-thin LPO-ALD coating exhibit continuous LPO phase injection after sintering, while maintaining an air stable and uniform LPO layer on the pellets, protecting the GSES from becoming H + / Li + exchange / Passivation film. The results show that, under the symmetric lithium battery structure, even at a high current density of 1.0 mA cm -2 , the interface resistance ASR of its lithium anode is negligible, and the cycle time exceeds 180 h. Under RT conditions, the CCD value of LPO @ LLZO reached a record 2.2mA cm −2 , which met the actual requirements of solid-state batteries. The solid-state lithium metal battery based on the Li / LPO @ LLZTO interface engineering has excellent rate performance and cycle stability.
2) The significant improvement of LPO @ LLZTO performance can be attributed to three aspects: (1) the flat top layer of LPO can conformally contact with the Li anode, resulting in uniform lithium plating / stripping; (2) the injected LPO fills the surface defects At the same time, the mechanical strength and lithium ion conductivity of the interconnected grain boundary structure are improved; (3) Li2O and Li3P rich SEI with negligible electronic conductivity is formed, and the lithium ion conductivity is high.
This interface engineering method combined with GSE grain boundary modification is an effective strategy to improve the SSLBs anode-electrolyte interface chemistry, and also provides a new design strategy for other types of solid-state batteries.
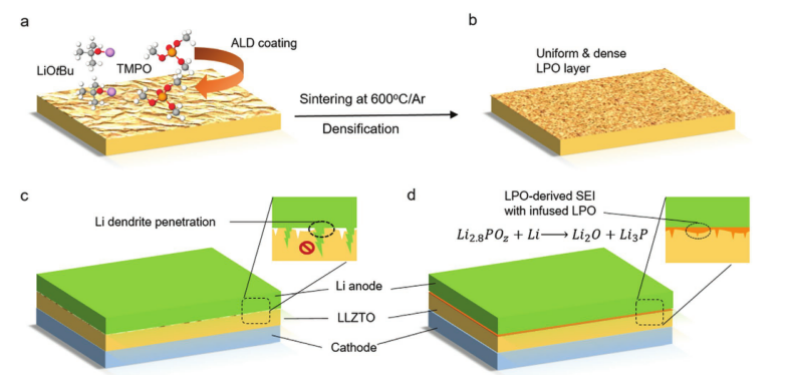
Tao Deng, et al, Tuning the Anode¨CElectrolyte Interface Chemistry for Garnet-Based Solid-State Li Metal Batteries, Adv. Mater. 2020
DOI: 10.1002 / adma.202000030
https://doi.org/10.1002/adma.202000030
6. Nano Today: Bio-inspired nano-scale electron / ion conduction network for room temperature all-solid sodium-sulfur batteries
A sulfur anode with a nano-scale electron / ion network is essential for the all-solid Na / S battery to achieve high energy density and long cycle life. However, it is very difficult to manufacture such structures using mechanical grinding or liquid phase reaction methods. In view of this, Yao Xiayin, a researcher at the Ningbo Institute of Materials Technology and Engineering , Chinese Academy of Sciences, and Professor Wang Chunsheng from the University of Maryland reported that by combining liquid phase reaction and mechanical grinding, a secondary part with micro-scale distribution of primary electron / ion channels and nano-scale distributions was fabricated Channel S-Na 3 SbS 4
- Previous£º Institute of Metallurg
- Next£º A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier