Quick View-Oxidation of MXene
QQ Academic Group: 1092348845
Detailed
Research Background
MXene of the unique properties, are widely used, involves a number of areas, typically obtained M X- ENE aqueous oxidation, degradation, so that the M X- ENE unique properties lost , this will introduce the M X- ENE oxide of related work , post Introduce M X ene antioxidant work again .
Literature 1
In situ environmental transmission electron microscopy study of oxidation of two-dimensional Ti 3 C 2 and formation of carbon-supported TiO 2
J. Mater. Chem. A, 2014, 2, 14339–14343 .
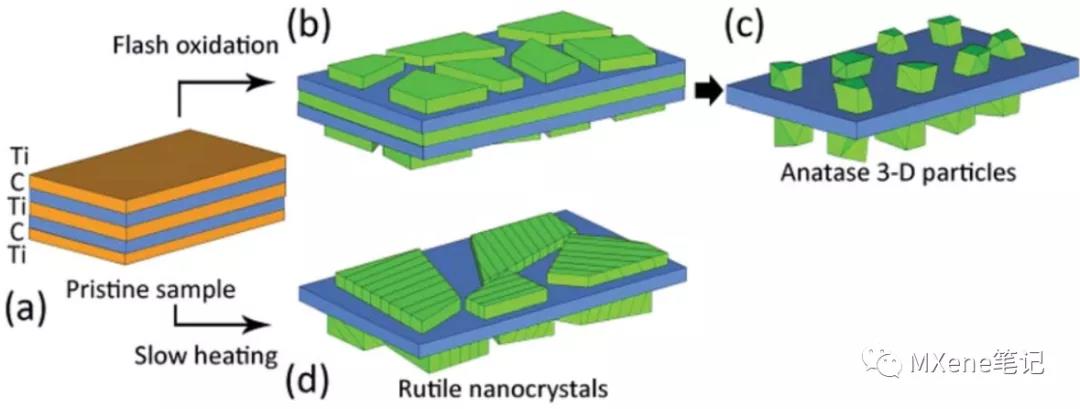
brief introduction
Dimensional MXene may be oxidized in air to form a carbon-supported Ti02 2 composites , in-situ TEM and Raman spectra show that, controlling the heating rate, temperature range, exposure time and other factors can be generated without isomorphous titania . Anatase nanoparticles can be obtained by rapid oxidation, and rutile flake crystals are formed when the heating rate is slow .
Literature 2
Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides
(MXenes)
Chem. Mater. 2017, 29, 4848−4856 .
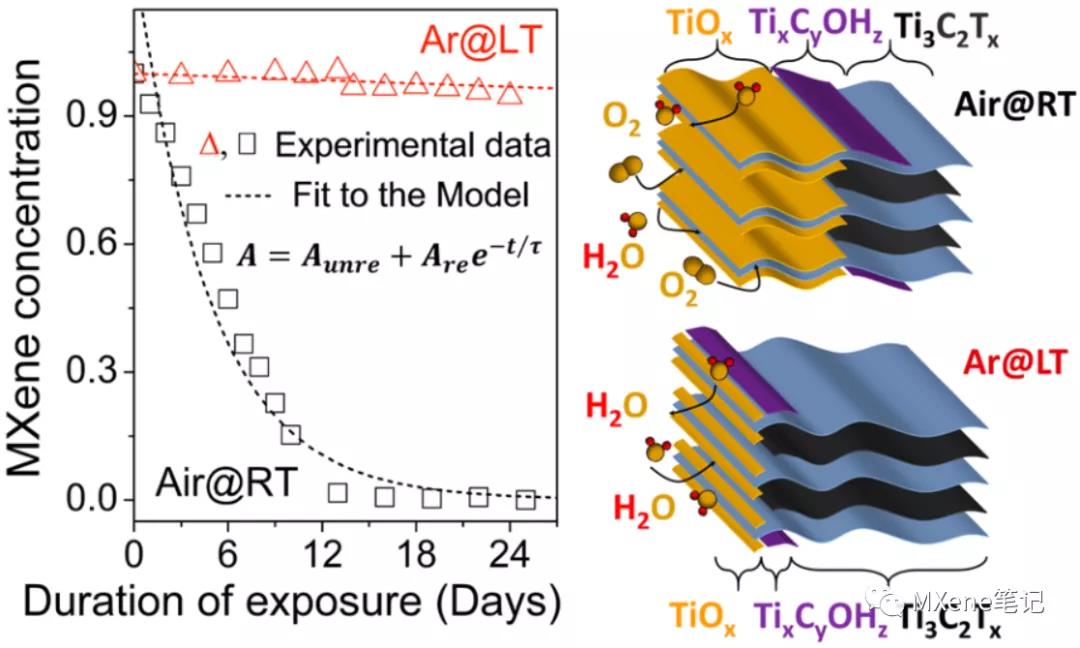
brief introduction
A systematic study of the oxidation of MXenes colloidal suspensions showed that dissolved oxygen, water, and high-temperature environments greatly accelerated degradation. Each degradation process is MXenes nano separate sheet among performed , sheets having a small diameter of MXenes nanosheet, the oxidation process faster by degradation of the relationship between the time constant and the sheet size can be from a UV- V IS situ Determine the size of the degraded nanosheets and vice versa. Another important conclusion is that MXenes oxygen onset to the sheet edge, and for inwardly , follow a single exponential decay behavior. MXenes colloids are more stable under argon, and therefore, an improved storage M X- Enes solution method, the MXene in the colloidal solution is stored Ar saturated aqueous solution , in the refrigerator, improve stability and time constants , to extend MXeneThe service life of the solution. The effectiveness of these methods in increasing the life of different MXene solutions is universal.
Literature 3
A comparative study on the oxidation of two-dimensional Ti 3 C 2 MXene structures in different Environments
J. Mater. Chem. A, 2018,6, 12733-12743 .
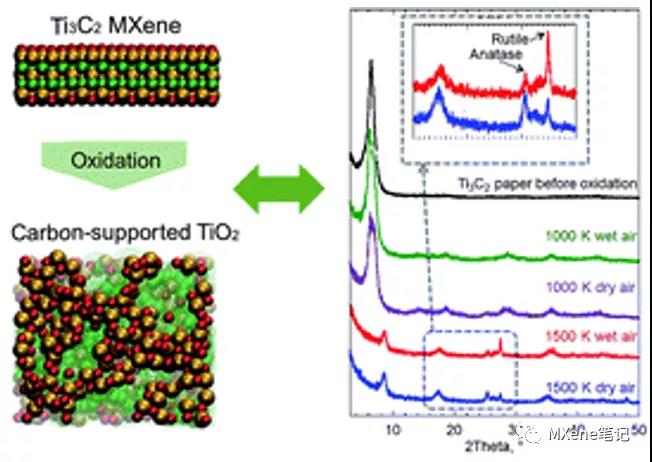
brief introduction
Through molecular dynamics simulations based on different storage media environments (hydrogen peroxide is composed of H 2 O 2 molecules, humid air is composed of oxygen and water molecules, and dry air is composed of only oxygen molecules) and temperature ( 1000 , 1500 , 2000 , 2500 And 3000 K ) . The article believes that the oxidation rate follows the following sequence: H 2 O 2 > humid air > dry air, and when the temperature rises, the oxidation rate will also increase. It is believed that dissolved oxygen in water is an important reason for oxidation , in contrast, High temperature will lead to the formation of cubic TiC .
Literature 4
Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal
Solutions
Inorg . Chem. 2019, 58, 1958−1966
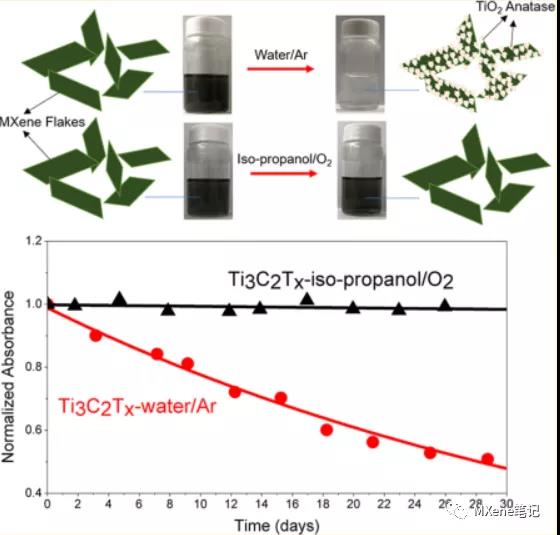
brief introduction
Herein proved by experiment , the water plays a role not only as a solvent , in MXenes degradation processes also play an important role, even under an inert atmosphere, in the presence of water it is detrimental to the MXene long-term stability. In aqueous solution in, MXene S hydrolysis of titanium carbide results in MXenes completely converted into titanium dioxide. As an application of the most widely studied and two Species MXenes material, using of Ti . 3 C 2 T X and of Ti 2 CT X conducted systematic studies , they have the same the elemental composition and chemical bonding , the difference is that only a single layer of a thickness ( . 5 th Atomic layer and 3 atomic layers ), Can be prepared by the same method , particularly suitable for different thickness of the impact properties of studies, we found 312 MAX phase than 211 MAX phase etching obtained MXenes better anti- oxidative stability, particularly two MXene S , of Ti . 3 C 2 T X and of Ti 2 CT X , water and isopropyl alcohol solvent have been in comparative studies , exposure to oxygen and an inert gas atmosphere. When both water and O 2 are present in the environment, the oxidation rates of the two MXenes are extremely fast, and the Ti 3 C 2 T x is 5 Days , Ti 2 CT x is 7 hours. When water or O 2 is alone, the degradation rate decreases, Ti 3 C 2 T x in water / Ar is 41 days , Ti 3 C 2 T x in isopropanol / O 2 is 2026 days , Ti 2 CT x in water / Ar for 2 days, Ti 2 CT x in isopropanol / O 2 is 7 days . Herein, data presentation and analysis clearly shows that a water instead of oxygen MXene degradation play a key role in the process , MXene saved need to isolate the water molecule in these results MXene preparation and storage has important significance .
Literature 5
An investigation into the factors governing the oxidation of two- dimensional Ti 3 C 2 MXene
Nanoscale, 2019, 11, 8387
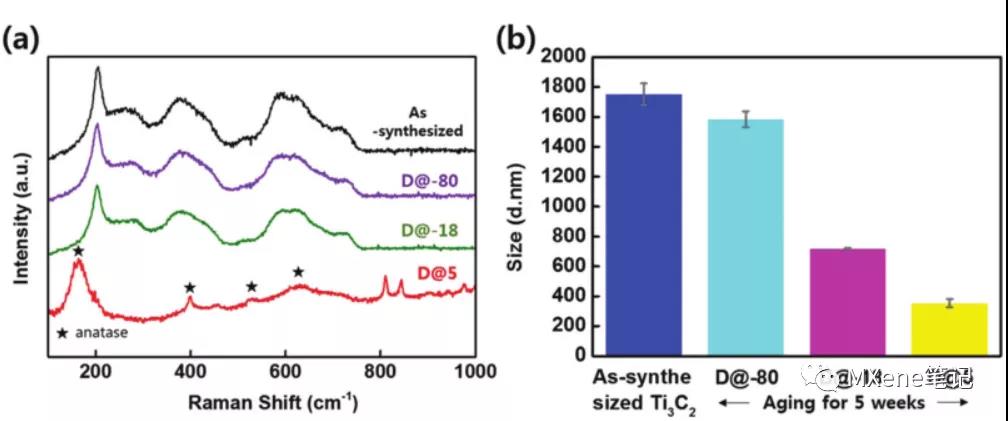
brief introduction
Previous research results indicate that water molecules affect oxidation, and the oxidation reaction rate is slower at low temperatures, based on these previous studies. This article discusses the main factors affecting the oxidation rate , determining the temperature and exposure of the water molecules is an important factor in the oxidation , of Ti . 3 C 2 T X oxidative stability of the obtained sheet well-controlled system has been improved in the temperature and water molecules , And put forward the principle about the preservation of M X enes . Found stored, of Ti . 3 C 2 T X MXene the aqueous solution may be maintained chemical stability exceeds 39 weeks ( temperature is - 80 degrees C ), a sufficiently low temperature, can stop the oxidation process. If Ti 3 C 2 T x flakes are dispersed in ethanol, even if stored in 5At degrees Celsius , the degradation process can also be significantly delayed. In both cases (low temperatures and an ethanol solvent) , the water can be reduced to the maximum extent of of Ti . 3 C 2 T X oxidative stability of the sheet of impact , can be used as MXenes ambient storage conditions. Suitable storage environment to extend the M X- Enes nanosheet degradation time , so that MXenes range of applications will be more extensive .
Source:
- Previous: JMCC: Wearable, long-l
- Next: Advanced Materials | A


 mxene academic
mxene academic
