Notes-how MXene resists oxidation
QQ Academic Group: 1092348845
Detailed
Research Background
MXene due to the unique physical and chemical properties, are widely used, it involves a number of areas, typically obtained MXene aqueous solution oxidation, degradation, such MXene nanosheet unique properties lost , how to avoid MXene oxide, except using an inert atmosphere, cold storage , non- aqueous solvent and other external storage media, if there is a chemical related to means of regulation, after all MXene has good hydrophilicity, provides many advantages in wet relevant chemical reaction more target applications from MXene starting solution , if The use of non-aqueous solvents such as isopropyl alcohol causes many inconveniences to the application of MXene . In view of this, this time will introduce MXene antioxidant work .
Literature 1
Antioxidants Unlock Shelf-Stable Ti3C2Tx (MXene) Nanosheet Dispersions
Matter 1, 513–526 .

brief introduction
The traditional means of preventing oxidation of MXenes mainly rely on low temperature ( refrigerator ) , creating an oxygen-free environment or filling with argon, etc., which is energy-protective and has no significant effect. As we all know, vitamin C, also known as L- ascorbic acid, is a common antioxidant and has a wide range of applications in food and pharmaceutical engineering. This paper attempts to introduce antioxidants into the MXenes nano-system to achieve the purpose of extending the shelf life of MXenes nanosheets by inhibiting the oxidation process . It was found that the chemical composition, morphology and colloidal stability of L- ascorbic acid introduced into the aqueous solution of Ti 3 C 2 T x nanosheets after 21 days storage compared with the MXenes nanosheets without L- ascorbic acid protection under the same conditions obvious difference. The conductivity of MXenes nanosheets protected by ascorbic acid is several orders of magnitude higher than that of unprotected ones. Through a method of molecular dynamics simulation ( ReaxFF ), the relationship between Ti 3 C 2 T x and antioxidantThe interaction between them was analyzed and it was found that the ascorbate anion can be adsorbed on the edge of the Ti 3 C 2 T x nanosheets, preventing the effect of water molecules on the MXenes nanosheets, thereby preventing their oxidation .
Literature 2
Edge Capping of 2D-MXene Sheets with Polyanionic Salts To Mitigate Oxidation in Aqueous Colloidal Suspensions
Angew. Chem. Int. Ed. 2019, 58, 12655–12660 .
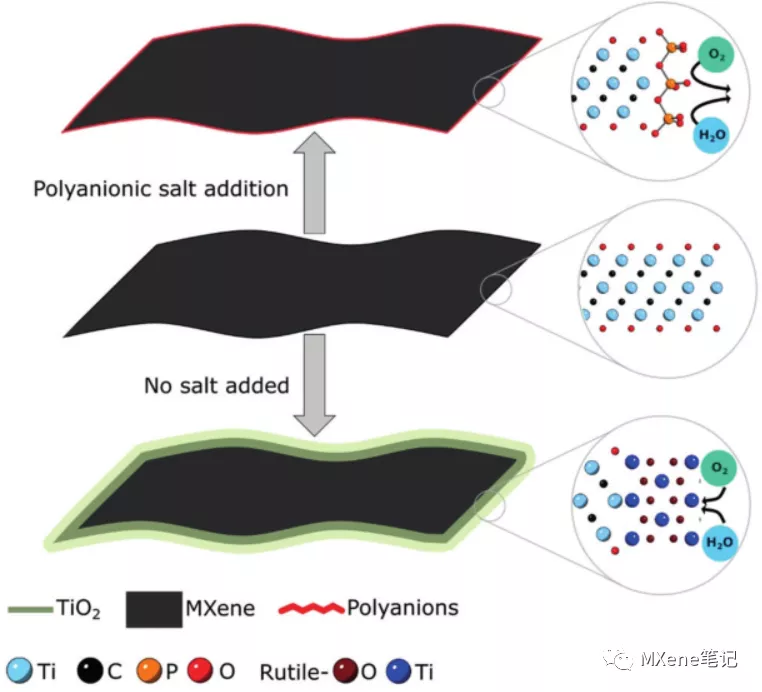
brief introduction
In this paper, it is found that, similar to clay, MXenes nanosheets are electronegative and their edges are positively charged. The oxidation process of MXenes also starts from the edge position and extends into the entire MXenes sheet . Our study showed, with polyanions, e.g. polyphosphate, a borate or poly polysilicate will MXene sheet edge of the package, i.e., polyanion adsorbed MXene edge, which can reduce the tendency to oxidize , even in aerobic placed in water For several weeks, its oxidation can also be inhibited. In addition, in some other important application areas, these salts are easily washed away without affecting their application. XPS analysis showed that the inhibition of the most obvious effect of polyphosphates, just 0.1M sufficient inhibition MXene oxidation at room temperature may be placed in water . 3 circumferential ( without inert gas protection, an aqueous solvent, at room temperature ) . Another advantage is the low cost phosphate and green, even on an industrial scale, but also the most promising long-term storage MXene colloidal most preferred selection .
Literature 3
Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application
Energy Storage Materials 25 (2020) 563–571 .
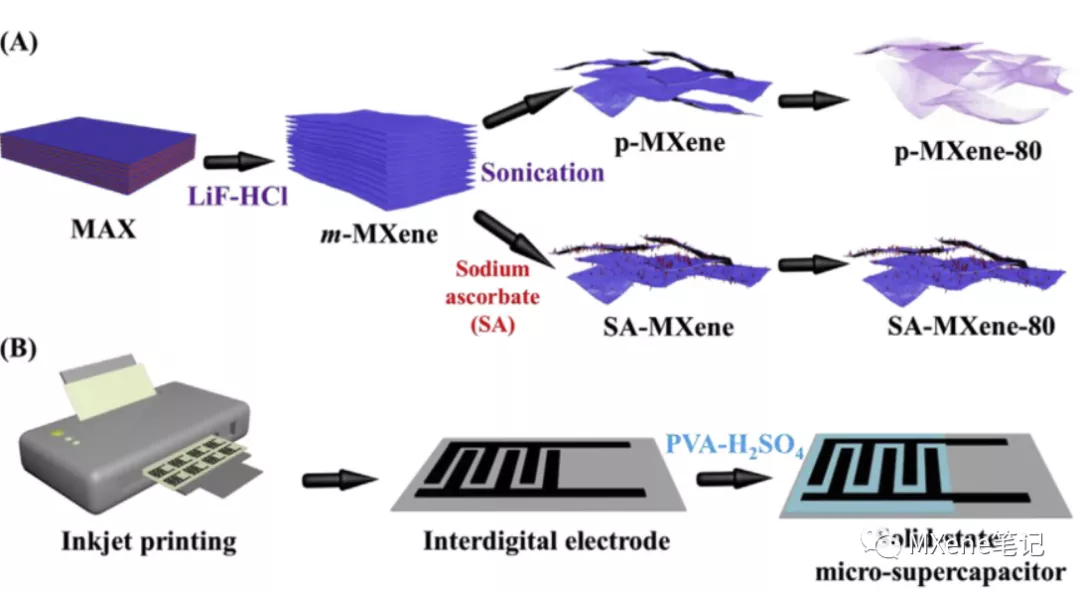
brief introduction
The low stability of MXenes in aqueous media limits their long-term storage and application. When ultrasonically peeling the multilayer MXenes, sodium ascorbate (SA) was added to prevent oxidation , and a SA-MXene dispersion was obtained. It was found that even at room temperature, when exposed to air, storage for 80 days would not produce oxidation , which greatly improved the shelf life of MXenes without affecting its conductivity, and applied research on micro supercapacitor electrodes, this work again The potential application of anionic groups to inhibit oxidation is highlighted .
Literature 4
Oxidation-resistant titanium carbide MXene films
J. Mater. Chem. A, 2020, 8,573

brief introduction
Compared to MXenes aqueous solution, MXenes film also has a wide range of applications, such as electromagnetic shielding, sensors, a transparent conductive film or the like . The physical properties of MXene thin-film field effect transistors will be destroyed in humid air. It is very meaningful to prepare MXenes thin films with stable physical and chemical properties under humid air and high temperature conditions . The research in this paper shows that through hydrogen atmosphere and short-time annealing at high temperature , the oxidation resistance of MXenes film can be increased , and for the oxidized MXenes film, partial recovery can be performed. During the hydrogen annealing process, the interlayer water and some functional groups volatilized, causing the (002) characteristic peak to move from a small angle to a large angle, and the interlayer spacing decreased from 12.4A to 10.1A . The treated film is also different from the original film in terms of contact angle. For the original film, a drop of water is sprinkled on it. The contact angle is 42 degrees at the beginning . After 10 seconds , the contact angle is 10 degrees, indicating that part of the water can penetrate MXenes sheet, and for the hydrogen annealing process MXenes film, the contact angle remains 42The degree of time can exceed 1 minute. Depth study shows that in the process of annealing, -OH, - F relative content decreased, - O relative content increased , which is also consistent with the findings once . With respect to the - OH and -F functional group, - O functional group having better thermal stability. In order to evaluate the stability of the relevant films, an impedance test was conducted. Due to the effects of oxidation , the curves of the untreated films and the low-temperature hydrogen-annealed films almost overlapped, and their resistance values increased geometrically with time, but less 15H , which exceeds 10 to 5 th order of magnitude , in the XRD see titania component in the spectrum, indicating that some oxidation. And 900 ° C high temperature treatment of the film, its value is relatively stable, after 2 days later, only 1.9 . Thus, high temperature hydrogen gas annealing imparting MXeneThe film is extremely resistant to oxidation , and even if immersed in water for 1 day , the resistance of the film hardly changes. SEM test shows that the micro-morphology of the two films is also very different. The surface of the hydrogen-annealed film is very smooth, without any significant distribution of particles. The untreated film has a rough surface and has obvious porous features in the enlarged image, indicating that the sheet has been destroyed and most of the surface has become oxides.
Literature 5
Covalent stabilization and functionalization of MXene via silylation reactions with improved surface properties
FlatChem 17 (2019) 100128
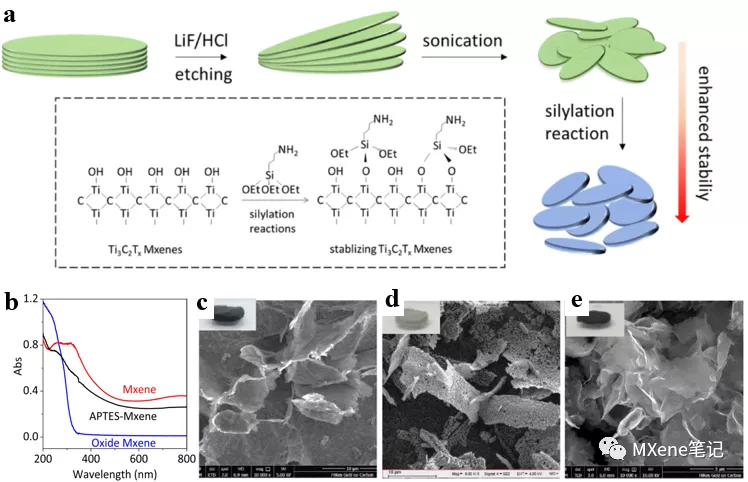
brief introduction
Chemical modification is considered to be an effective method to control the surface characteristics of materials. As a common coupling agent, silylating agents have been widely used to adjust the interface and surface properties of various materials . Thanks to the above-described inspiration, previously reported in LiF and HCl after the etching method and a lift-off process subsequent ultrasound, paper MXene was modified layered MXenes nanosheet various silylating agent with MXenes in ethanol Reaction ( figure a) . The degradation degree of MXenes can be measured by UV-Vis measurement according to the change in absorbance of the sample (Figure b ). Oxidation at room temperature for 6 days, the obtained oxidation of MXene S , fresh MXene S compared at 320-800nm decreased absorbance wavelength. It is worth noting that the absorption strength of MXene s ( APTES-MXene ) treated with silylation is different from fresh MXene sThe absorption intensity is almost the same , indicating that the oxidation process is indeed inhibited by surface modification. After freeze- drying the relevant MXenes solution, the structural integrity of MXenes can also be detected by SEM (Figure c- e ). Modified MXene S with fresh MXene S identical, humidity of 100 through% atmosphere of 15 post-oxidation treatment days, it showed a significant and uniform nano sheet. However, due to oxidation, unmodified MXene s becomes rough. Related illustrations are freeze-dried samples of photo oxidation after MXene S is white, this may be Ti02 2 formed as a result .
Note :
The conclusions of the first three articles are similar, even in a broad sense: the polyanion group is adsorbed on the edge of the MXenes sheet ( because the MXenes edge is positively charged, it can be adsorbed or even encapsulated ) , preventing the action of water molecules, thereby preventing MXenes Of oxidation .
① MXenes degradation process in each MXenes nano separate sheet among performed, sheets having a small diameter of MXenes nanosheet, the oxidation process is much faster .
② The oxidation of MXenes starts at the edge of the sheet and then proceeds inward, following a single exponential decay behavior. In the chemical formula M n + 1 X n , the smaller the value of n, the easier it is to oxidize.
③ Store the MXene colloid solution in Ar saturated aqueous solution and refrigerate it in the refrigerator to improve its stability and time constant .
④ water instead of oxygen MXene plays a key role in the degradation process, MXene saving the required isolation of water molecules ( spacer does not mean that water molecules in the non-aqueous solvent ) .
⑤The surface of the conventionally prepared MXenes nanosheets is electronegative, with positive charges on the edges, and the addition of polyanionic groups can prevent the oxidation of MXenes .
Source: M X ene notes
- Previous: Preparation-From a few
- Next: MXene breakthrough: Na


 mxene academic
mxene academic
