Professor Han Weiqiang of Zhejiang University: Pillar-less MXenes--Solving Reunion Problems to Improve Lithium Ion Storage Performance
QQ Academic Group: 1092348845
Detailed
Research Background
Due to the unique physical and chemical properties, the research of the new two-dimensional material MXenes has received more and more attention, involving energy storage, catalysis, electromagnetic shielding and other fields. Among the many properties of MXenes, the two characteristics of large interlayer spacing and surface electronegativity are the unique points that distinguish MXenes from other two-dimensional materials. The layer spacing of MXenes is large and flexible, and the MXenes with increased layer spacing are visually called pillar MXenes. When MXenes is used directly as a negative electrode material for lithium batteries, its own capacity is relatively low, and its electrochemical performance can be greatly improved by the way of pillaring compound with other active material materials. In the past few years, the research on MXenes‘ pillars has basically stayed in different active substances, different pre-pillaring reagents, and the few layers of MXenes nanosheets are easy to agglomerate. There has been no research report on pillared few layers of MXenes.
Highlights of this article
1. Through the ammonium ion method, the problem of agglomeration of few layers of MXenes is solved, and a composite of tin oxide pillared few layers of Ti3C2Tx MXenes is prepared.
2. Due to the pillar effect, this composite material has excellent lithium ion storage performance. At a current density of 2A/g, after 1200 cycles, the specific capacity is 1016 mAh/g. At a current density of 5A/g, the specific capacity can be Up to 680 mAh/g.
brief introduction
Based on the unique properties of MXenes (large interlayer spacing and electronegativity on the surface), the research team of Han Weiqiang from the School of Materials Science and Engineering of Zhejiang University solved the problem of agglomeration of MXenes through the ammonium ion method, and obtained the first few layers of tin oxide pillars The composite material of Ti3C2Tx MXenes is denoted as STCT. Thanks to the low layer structure and the pillar effect, the STCT composite exhibits excellent electrochemical performance. At a current density of 2 A/g, after 1,200 cycles, the specific capacity of the composite can reach 1016 mAh/g. At a high current density of 5 A/g, the specific capacity can be stabilized at 680 mAh/g, indicating good rate performance, better than the reported pillared MXenes system (in a multi-layer state, including a higher specific capacity than itself) Compared with MXenes such as low molecular weight V2C, Ti2C, etc.), it strongly illustrates the application potential and advantages of the pillared few-layer MXenes composite material in the field of electrochemical energy storage. Since it is based on the nature of MXenes, the ammonium ion method mentioned in this article and the support of a few layers of MXenes can be easily extended to other systems.
Graphic guide
I. Preparation and characterization of composite materials
The preparation process of related materials is shown in Figure 1. Due to the adsorption of functional groups such as -F and -OH on the surface, the surface of the MXenes nanosheets exhibits electronegativity, and the electrostatic repulsion between the MXenes nanosheets causes the few layers of MXenes nanoparticles after peeling. The tablets can exist stably in the aqueous solution. By introducing the ammonium ion method, the electrostatic equilibrium state of the aqueous solution system can be destroyed, and the process of electrostatic precipitation occurs. The flocculated product is freeze-dried and annealed in an Ar atmosphere (removing volatile) Ammonium salt, such as ammonium bicarbonate), you can get a few layers of MXenes nanosheets, starting from 500 times the SEM image, zoom in and observe, you can see the obvious nanosheets, no agglomeration phenomenon, indicating that through the ammonium ion Method can solve the reunion problem of MXenes. According to previous reports, the prepared few layers of Ti3C2Tx MXenes nanosheets are pre-pillared with CTAB to increase the interlayer spacing, and then tin salts are introduced. Through charge exchange, the tin-based nanocomposite pillared few layers can be prepared Composite material (STCT) of Ti3C2Tx MXenes.
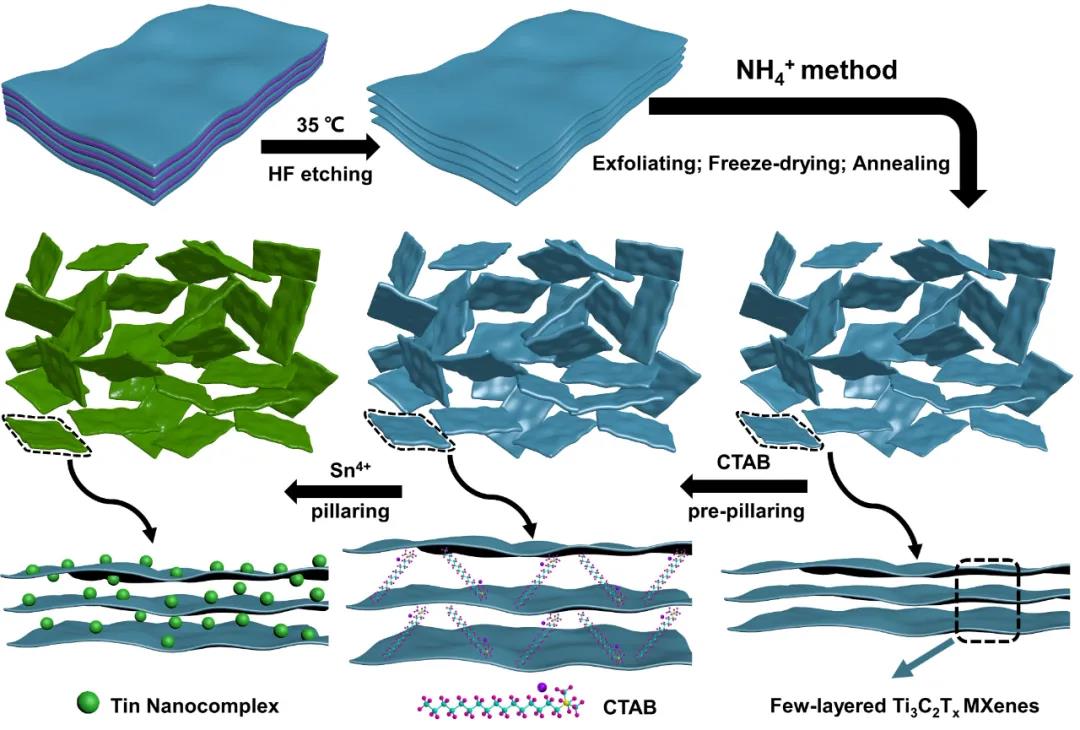
Figure 1. Schematic diagram of the preparation of a few layers of MXenes nanosheets and STCT composite materials.
From the XRD patterns of related materials (Figure 2), it can be seen that compared with the raw material MAX phase Ti3AlC2, after HF acid etching, the main peak (104) at 39 degrees disappears, and the (002) characteristic peak of the MXenes layered material Strengthening and moving to a small angle shows that the aluminum layer in MAX is completely etched. After the surfactant CTAB pre-pillaring, the (002) characteristic diffraction peak reached 4 degrees, which corresponds to the embedding of CTAB molecules, which increases the interlayer spacing of MXenes. After passing through the Sn pillar, the characteristic peak moves to the right, but the angle is much smaller than the characteristic peak angle of MXenes alone, indicating that some atomic-level tin-based nanocomposites have been pillared to a few layers of Ti3C2Tx MXenes.
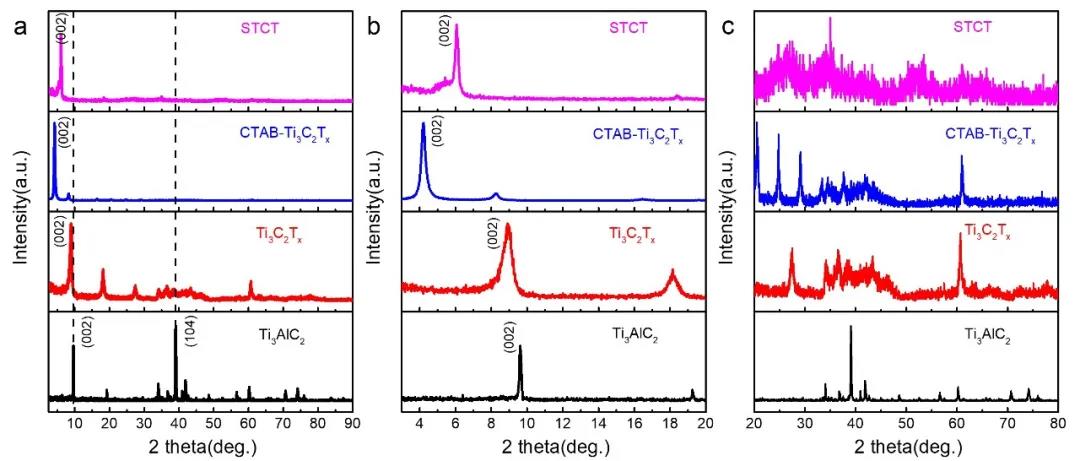
Figure 2. XRD patterns of related materials: raw material MAX phase Ti3AlC2, etched Ti3C2Tx MXenes, CTAB pre-pillared MXenes (CTAB-Ti3C2Tx), the final composite material STCT. (a) 3 degrees to 90 degrees, (b) 3 degrees to 20 degrees, (c) 20 degrees to 90 degrees.
Figure 3 is the microstructure characterization of the prepared few-layer MXenes nanosheets and the final STCT composite material. In the AFM test results, the thickness of the MXenes nanosheets is 1-3 nm, and the corresponding nanosheets are 1-3 layers, indicating that the successful preparation has less Layer of MXene nanosheets. In the SEM and TEM images, you can see the obvious layer structure, especially from the multiple SEM step by step, there is no agglomeration phenomenon of MXene nanosheets, indicating that the method of ammonium ion assisted can solve the few layers of MXenes nanometers The problem of reunion. After pillaring, the surface of the STCT composite becomes rough, and the particles scattered on the surface of MXenes can be seen in the TEM image, corresponding to the tin-based nanocomposite. After pillaring, the layer spacing of MXenes becomes larger, from 1.18-1.62 nm Unequal, indicating that some atomic-scale tin-based nanocomposites are pillared between the few layers of MXenes nanosheets, and some 3-5nm-sized tin-based nanocomposites are adsorbed on the surface of MXenes nanosheets, or sandwiched between 2 MXenes Between nanosheets. In the process of preparing composite materials, CTAB as a surfactant can prevent the agglomeration and growth of Sn-based particles, and can also pre-pillar MXenes, adjust the interlayer spacing, embed part of the active material Sn-based particles, MXenes with larger interlayer distance In addition, it can further suppress the growth of Sn-based particles and provide more space for lithium ion storage.
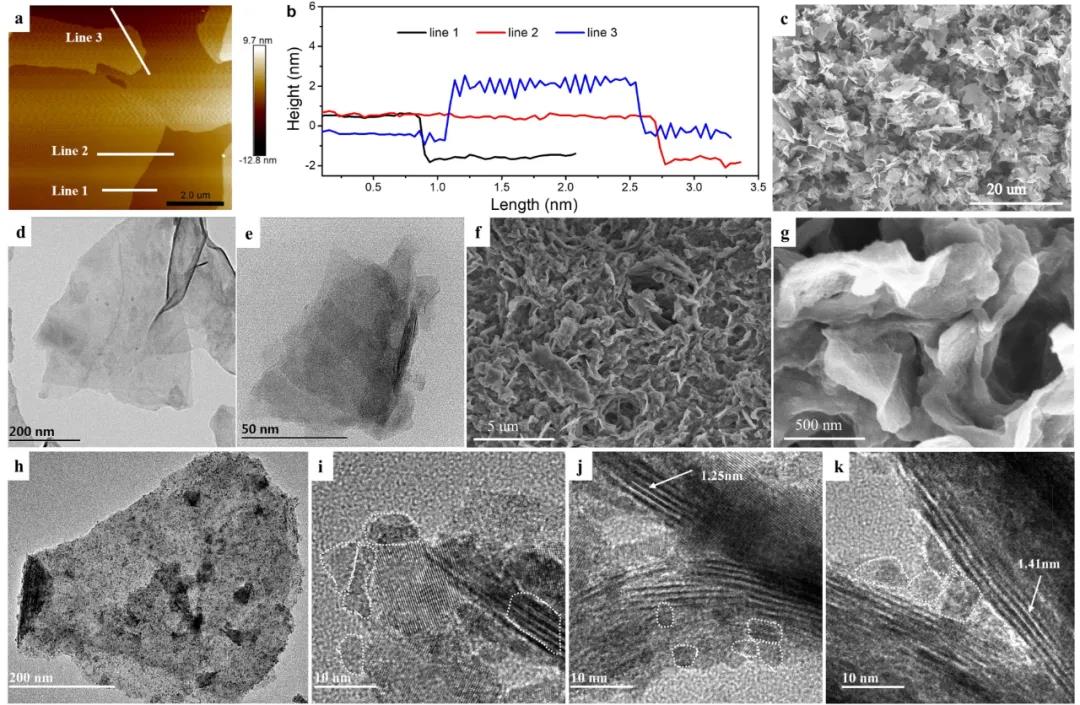
Figure 3. Microscopic characterization of low-layer MXenes nanosheets and STCT composites: (a-b) AFM test of low-layer MXenes, (c-e) SEM and TEM of low-layer MXenes, (f-k) SEM and TEM of STCT composites.
Through XPS test analysis, it is found that the fine spectrum of Sn 3d is located at 495.4 and 487.0 eV, corresponding to Sn 3d3/2 and 3d5/2, respectively. After the peak splitting process, it was determined that Sn(2+) and Sn(4+) nanocomposites existed approximately in a mass ratio of 1:2, corresponding to the oxide form of Sn. In Ti3C2Tx and STCT composites, Ti 2p is composed of four peaks, two peaks with higher binding energy correspond to Ti 2p1/2, and the other two correspond to 2p3/2. After peak splitting, Ti 2p is fitted to Ti(2+), Ti(3+), Ti-O and Ti-C peaks. Compared with the 3 peaks of O 1s of Ti3C2Tx, a peak corresponding to the Sn–O bond (530.4 eV) appears in the STCT composite, indicating that the tin oxide is bonded to the oxygen-containing group on the surface of MXenes (for example- OH and -O), the Sn-O-Ti chemical bond is formed at the interface, which helps to maintain the stability of the structure during the cycle and prevent the active material from falling off from the conductive substrate, thereby ensuring stable electrochemical performance.
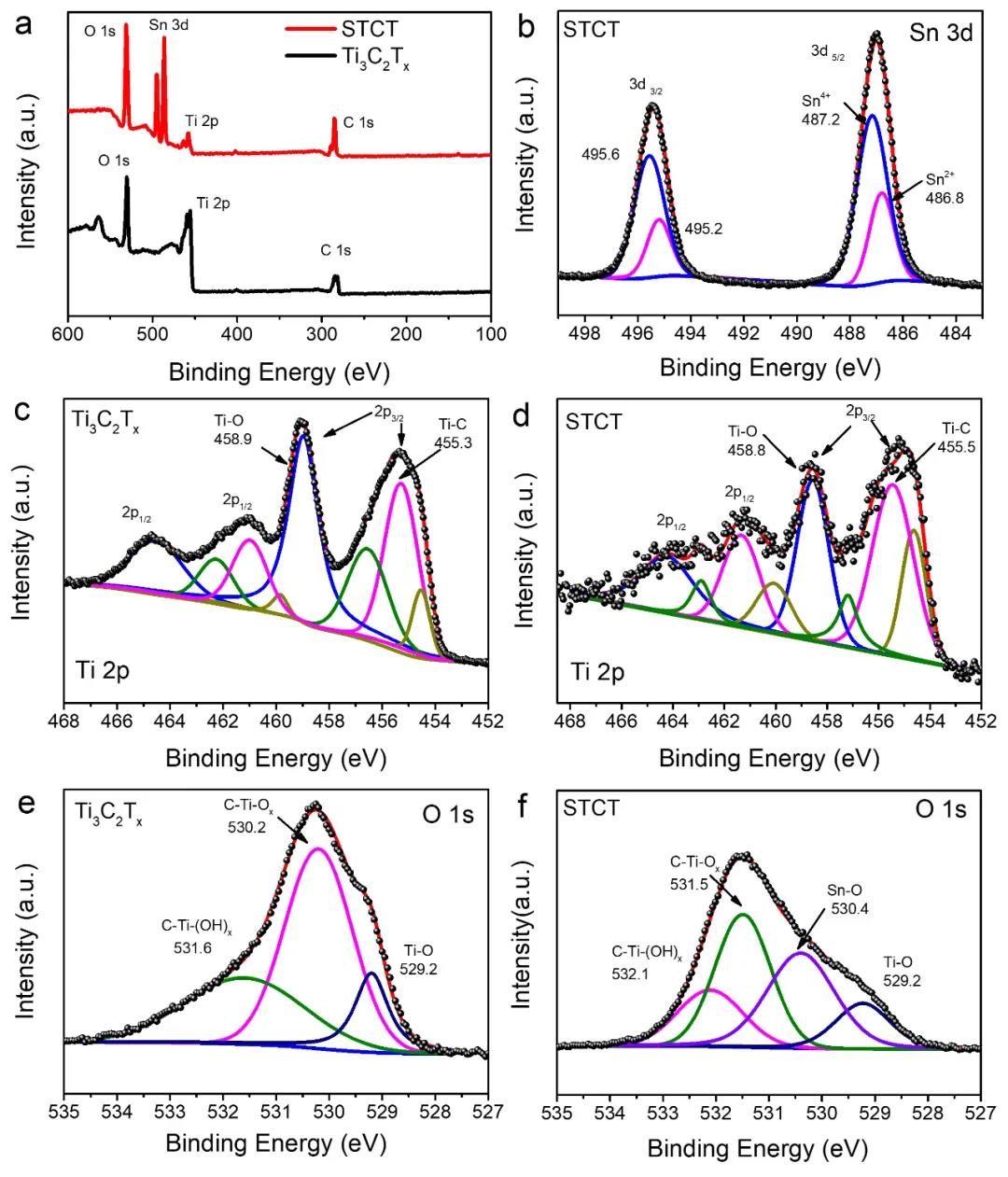
Figure 4. XPS test of Ti3C2Tx nanosheets and final composite STCT: (a) full spectrum, (b) Sn 3d fine spectrum in STCT, (c-d) Ti2p fine spectrum, (e-f) O 1s fine spectrum.
II STCT electrochemical performance test and analysis
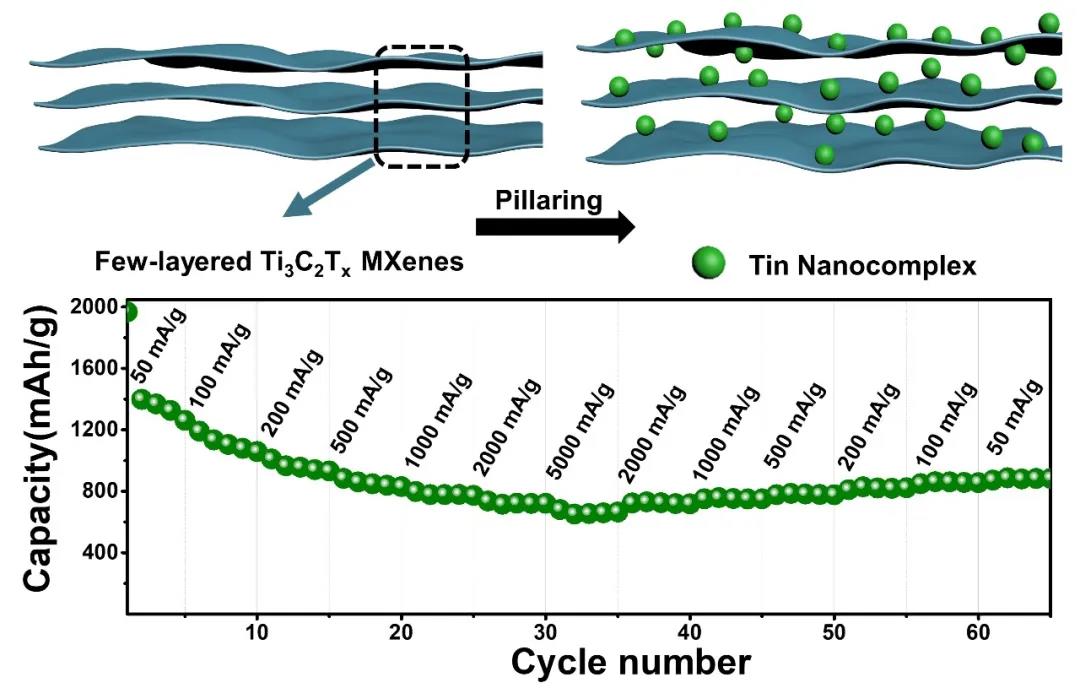
Assemble a 2032 button cell and conduct electrochemical performance tests on the STCT composite material. In the CV curve, during the first negative sweep, a relatively wide peak appeared at about 0.8 V, corresponding to the formation of the SEI film, as well as Sn oxide and Li+ The reduction reaction forms Sn and Li2O, the latter reaction is partially reversible, so in the subsequent periodic cycle, you can see a clear peak at 0.92 V. The peak below 0.5V is due to the alloying reaction of Sn and Li+ to form LixSn and the Ti3C2Tx matrix containing functional groups on the surface to adsorb lithium ions, which is partly reversible and leads to a serious decrease in the specific capacity of the first discharge. During the positive scan, the main peak is about 0.54 V, which belongs to the dealloying reaction of LixSn. In addition, the two secondary peaks around 1.2 and 1.9 V correspond to the reactions from Sn to SnO and SnO2. From the second cycle, a good curve The overlap indicates the good reversibility of the system. The first discharge capacity can reach 1892.4 mAh/g, corresponding to a Coulomb efficiency of 68.3%. The irreversible capacity loss comes from the inevitably formed SEI film, the irreversible conversion of tin oxide to Sn, and the adsorption of Li+ on the layered Ti3C2Tx surface. Thanks to the low-layer structure, the STCT composite material exhibits excellent rate performance. At a current density of 5 A/g, the capacity can reach 680 mAh/g. When the current density is reduced to 500 mA/g again, it can still be obtained. Up to 793.3 mAh/g specific capacity. In the long cycle test of 500 mA/g and 2000 mA/g current density, there will be a phenomenon of capacity increase in the later period. The greater the current density, the more obvious the phenomenon of capacity increase, due to the insertion and extraction of ions during the cycle And the volume change of the Sn-based active material makes the interlayer distance of MXenes further increase, releasing additional storage space for lithium ions.
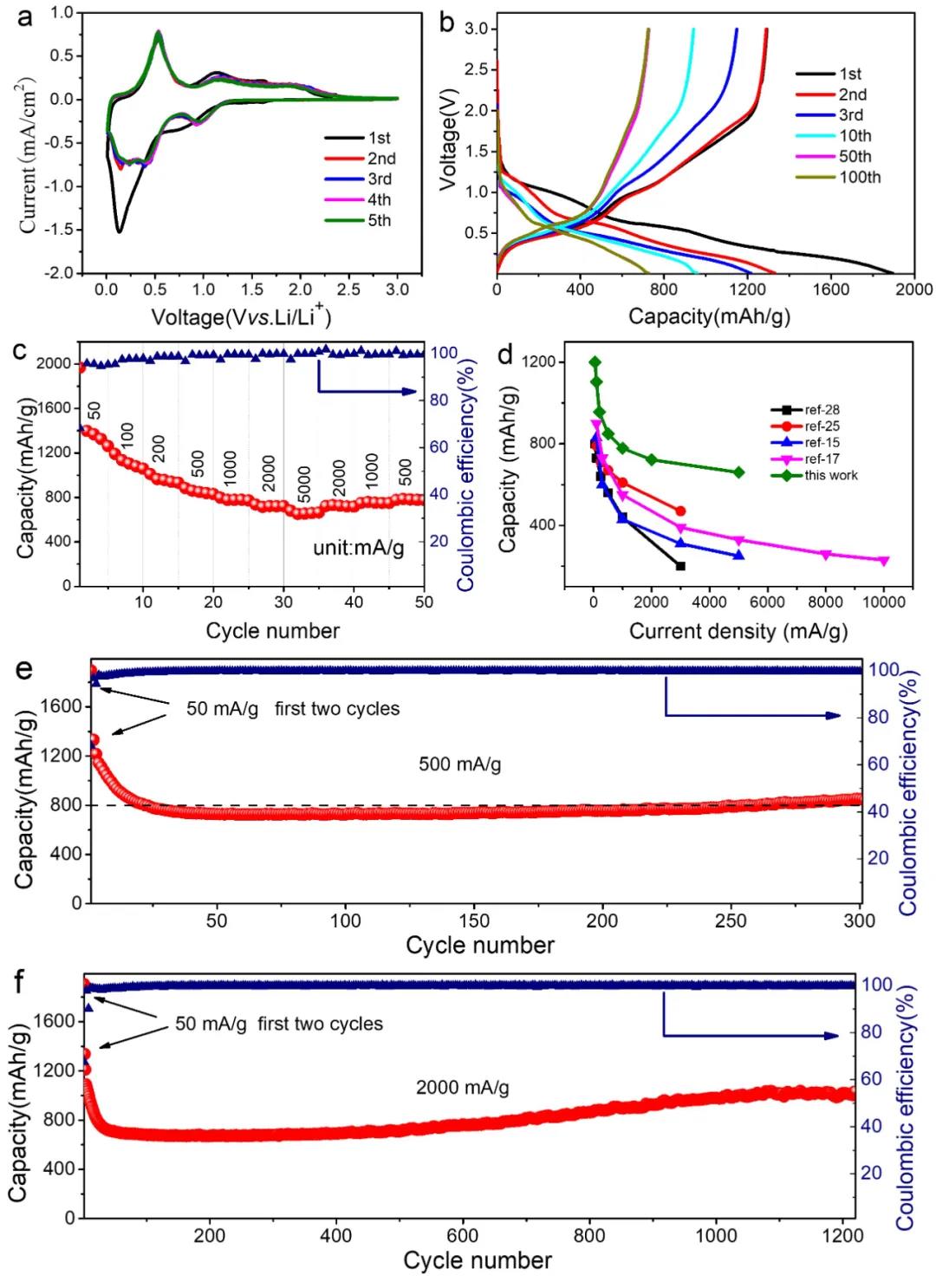
Figure 5. STCT composite electrochemical performance test: (a) CV curve (0.1mV/s), (b) charge-discharge curve at 500mA/g, (c) rate test, (d) rate of related pillar MXenes Performance comparison, (ef) cycle performance at 500 mA/g and 2000 mA/g.
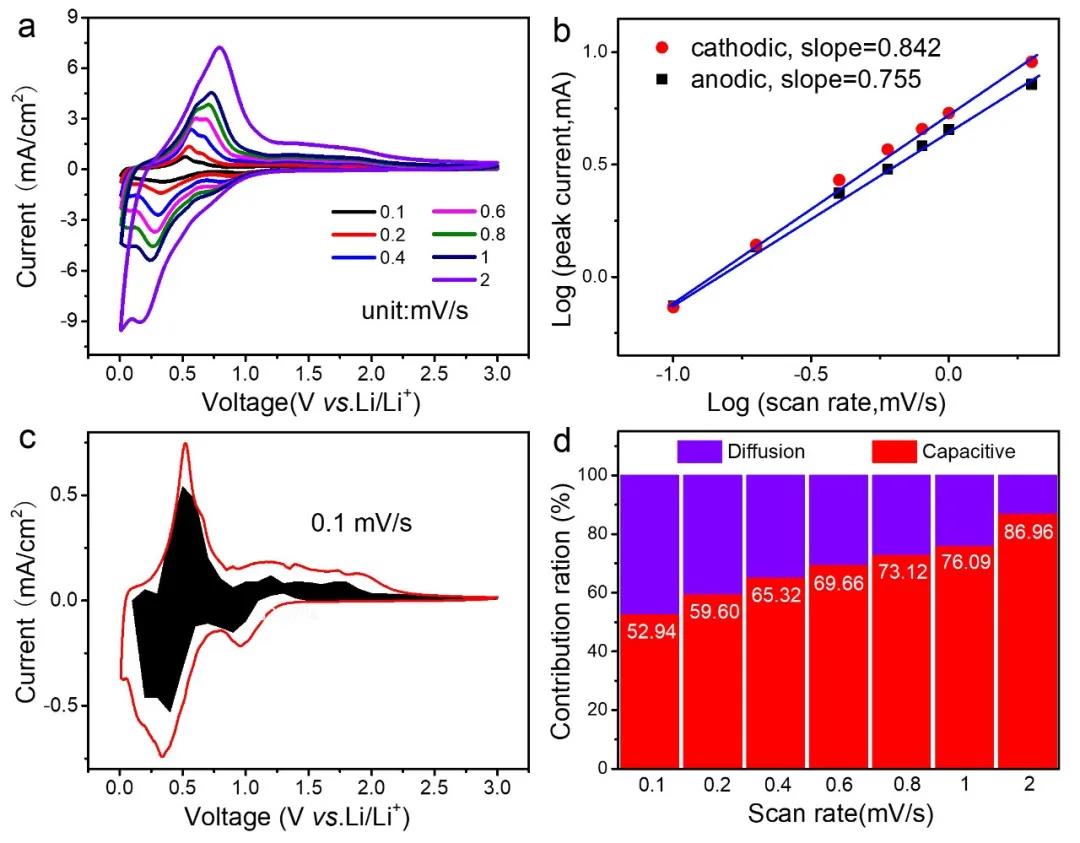
Because MXenes itself is a capacitive material, it is necessary to analyze the capacitance ratio. In the CV curves of different sweep speeds, as the sweep speed increases, the polarization increases. The fitting analysis of logi and logv shows that the slope Greater than 0.5, between 0.5 and 1, the contribution of capacitance cannot be ignored. The high lithium storage capacity of the STCT composite material contains a relatively high proportion of capacitance contribution, and it increases with the increase of the scanning speed, so it has excellent rate performance, and the composite material itself is bonded with a stable microstructure to make it have Good cycle stability.
Due to the unique physical and chemical properties, the research of the new two-dimensional material MXenes has received more and more attention, involving energy storage, catalysis, electromagnetic shielding and other fields. Among the many properties of MXenes, the two characteristics of large interlayer spacing and surface electronegativity are the unique points that distinguish MXenes from other two-dimensional materials. The layer spacing of MXenes is large and flexible, and the MXenes with increased layer spacing are visually called pillar MXenes. When MXenes is used directly as a negative electrode material for lithium batteries, its own capacity is relatively low, and its electrochemical performance can be greatly improved by the way of pillaring compound with other active material materials. In the past few years, the research on MXenes‘ pillars has basically stayed in different active substances, different pre-pillaring reagents, and the few layers of MXenes nanosheets are easy to agglomerate. There has been no research report on pillared few layers of MXenes.

Partial Atomic Tin Nanocomplex Pillared Few‑Layered Ti3C2Tx MXenes for Superior Lithium‑Ion Storage
Shunlong Zhang, Hangjun Ying, Bin Yuan, Renzong Hu, Wei‑Qiang Han*
Nano-Micro Lett.(2020)12:78
https://doi.org/10.1007/s40820-020-0405-7
Highlights of this article
1. Through the ammonium ion method, the problem of agglomeration of few layers of MXenes is solved, and a composite of tin oxide pillared few layers of Ti3C2Tx MXenes is prepared.
2. Due to the pillar effect, this composite material has excellent lithium ion storage performance. At a current density of 2A/g, after 1200 cycles, the specific capacity is 1016 mAh/g. At a current density of 5A/g, the specific capacity can be Up to 680 mAh/g.
brief introduction
Based on the unique properties of MXenes (large interlayer spacing and electronegativity on the surface), the research team of Han Weiqiang from the School of Materials Science and Engineering of Zhejiang University solved the problem of agglomeration of MXenes through the ammonium ion method, and obtained the first few layers of tin oxide pillars The composite material of Ti3C2Tx MXenes is denoted as STCT. Thanks to the low layer structure and the pillar effect, the STCT composite exhibits excellent electrochemical performance. At a current density of 2 A/g, after 1,200 cycles, the specific capacity of the composite can reach 1016 mAh/g. At a high current density of 5 A/g, the specific capacity can be stabilized at 680 mAh/g, indicating good rate performance, better than the reported pillared MXenes system (in a multi-layer state, including a higher specific capacity than itself) Compared with MXenes such as low molecular weight V2C, Ti2C, etc.), it strongly illustrates the application potential and advantages of the pillared few-layer MXenes composite material in the field of electrochemical energy storage. Since it is based on the nature of MXenes, the ammonium ion method mentioned in this article and the support of a few layers of MXenes can be easily extended to other systems.
Graphic guide
I. Preparation and characterization of composite materials
The preparation process of related materials is shown in Figure 1. Due to the adsorption of functional groups such as -F and -OH on the surface, the surface of the MXenes nanosheets exhibits electronegativity, and the electrostatic repulsion between the MXenes nanosheets causes the few layers of MXenes nanoparticles after peeling. The tablets can exist stably in the aqueous solution. By introducing the ammonium ion method, the electrostatic equilibrium state of the aqueous solution system can be destroyed, and the process of electrostatic precipitation occurs. The flocculated product is freeze-dried and annealed in an Ar atmosphere (removing volatile) Ammonium salt, such as ammonium bicarbonate), you can get a few layers of MXenes nanosheets, starting from 500 times the SEM image, zoom in and observe, you can see the obvious nanosheets, no agglomeration phenomenon, indicating that through the ammonium ion Method can solve the reunion problem of MXenes. According to previous reports, the prepared few layers of Ti3C2Tx MXenes nanosheets are pre-pillared with CTAB to increase the interlayer spacing, and then tin salts are introduced. Through charge exchange, the tin-based nanocomposite pillared few layers can be prepared Composite material (STCT) of Ti3C2Tx MXenes.

Figure 1. Schematic diagram of the preparation of a few layers of MXenes nanosheets and STCT composite materials.
From the XRD patterns of related materials (Figure 2), it can be seen that compared with the raw material MAX phase Ti3AlC2, after HF acid etching, the main peak (104) at 39 degrees disappears, and the (002) characteristic peak of the MXenes layered material Strengthening and moving to a small angle shows that the aluminum layer in MAX is completely etched. After the surfactant CTAB pre-pillaring, the (002) characteristic diffraction peak reached 4 degrees, which corresponds to the embedding of CTAB molecules, which increases the interlayer spacing of MXenes. After passing through the Sn pillar, the characteristic peak moves to the right, but the angle is much smaller than the characteristic peak angle of MXenes alone, indicating that some atomic-level tin-based nanocomposites have been pillared to a few layers of Ti3C2Tx MXenes.

Figure 2. XRD patterns of related materials: raw material MAX phase Ti3AlC2, etched Ti3C2Tx MXenes, CTAB pre-pillared MXenes (CTAB-Ti3C2Tx), the final composite material STCT. (a) 3 degrees to 90 degrees, (b) 3 degrees to 20 degrees, (c) 20 degrees to 90 degrees.
Figure 3 is the microstructure characterization of the prepared few-layer MXenes nanosheets and the final STCT composite material. In the AFM test results, the thickness of the MXenes nanosheets is 1-3 nm, and the corresponding nanosheets are 1-3 layers, indicating that the successful preparation has less Layer of MXene nanosheets. In the SEM and TEM images, you can see the obvious layer structure, especially from the multiple SEM step by step, there is no agglomeration phenomenon of MXene nanosheets, indicating that the method of ammonium ion assisted can solve the few layers of MXenes nanometers The problem of reunion. After pillaring, the surface of the STCT composite becomes rough, and the particles scattered on the surface of MXenes can be seen in the TEM image, corresponding to the tin-based nanocomposite. After pillaring, the layer spacing of MXenes becomes larger, from 1.18-1.62 nm Unequal, indicating that some atomic-scale tin-based nanocomposites are pillared between the few layers of MXenes nanosheets, and some 3-5nm-sized tin-based nanocomposites are adsorbed on the surface of MXenes nanosheets, or sandwiched between 2 MXenes Between nanosheets. In the process of preparing composite materials, CTAB as a surfactant can prevent the agglomeration and growth of Sn-based particles, and can also pre-pillar MXenes, adjust the interlayer spacing, embed part of the active material Sn-based particles, MXenes with larger interlayer distance In addition, it can further suppress the growth of Sn-based particles and provide more space for lithium ion storage.

Figure 3. Microscopic characterization of low-layer MXenes nanosheets and STCT composites: (a-b) AFM test of low-layer MXenes, (c-e) SEM and TEM of low-layer MXenes, (f-k) SEM and TEM of STCT composites.
Through XPS test analysis, it is found that the fine spectrum of Sn 3d is located at 495.4 and 487.0 eV, corresponding to Sn 3d3/2 and 3d5/2, respectively. After the peak splitting process, it was determined that Sn(2+) and Sn(4+) nanocomposites existed approximately in a mass ratio of 1:2, corresponding to the oxide form of Sn. In Ti3C2Tx and STCT composites, Ti 2p is composed of four peaks, two peaks with higher binding energy correspond to Ti 2p1/2, and the other two correspond to 2p3/2. After peak splitting, Ti 2p is fitted to Ti(2+), Ti(3+), Ti-O and Ti-C peaks. Compared with the 3 peaks of O 1s of Ti3C2Tx, a peak corresponding to the Sn–O bond (530.4 eV) appears in the STCT composite, indicating that the tin oxide is bonded to the oxygen-containing group on the surface of MXenes (for example- OH and -O), the Sn-O-Ti chemical bond is formed at the interface, which helps to maintain the stability of the structure during the cycle and prevent the active material from falling off from the conductive substrate, thereby ensuring stable electrochemical performance.

Figure 4. XPS test of Ti3C2Tx nanosheets and final composite STCT: (a) full spectrum, (b) Sn 3d fine spectrum in STCT, (c-d) Ti2p fine spectrum, (e-f) O 1s fine spectrum.
II STCT electrochemical performance test and analysis
Assemble a 2032 button cell and conduct electrochemical performance tests on the STCT composite material. In the CV curve, during the first negative sweep, a relatively wide peak appeared at about 0.8 V, corresponding to the formation of the SEI film, as well as Sn oxide and Li+ The reduction reaction forms Sn and Li2O, the latter reaction is partially reversible, so in the subsequent periodic cycle, you can see a clear peak at 0.92 V. The peak below 0.5V is due to the alloying reaction of Sn and Li+ to form LixSn and the Ti3C2Tx matrix containing functional groups on the surface to adsorb lithium ions, which is partly reversible and leads to a serious decrease in the specific capacity of the first discharge. During the positive scan, the main peak is about 0.54 V, which belongs to the dealloying reaction of LixSn. In addition, the two secondary peaks around 1.2 and 1.9 V correspond to the reactions from Sn to SnO and SnO2. From the second cycle, a good curve The overlap indicates the good reversibility of the system. The first discharge capacity can reach 1892.4 mAh/g, corresponding to a Coulomb efficiency of 68.3%. The irreversible capacity loss comes from the inevitably formed SEI film, the irreversible conversion of tin oxide to Sn, and the adsorption of Li+ on the layered Ti3C2Tx surface. Thanks to the low-layer structure, the STCT composite material exhibits excellent rate performance. At a current density of 5 A/g, the capacity can reach 680 mAh/g. When the current density is reduced to 500 mA/g again, it can still be obtained. Up to 793.3 mAh/g specific capacity. In the long cycle test of 500 mA/g and 2000 mA/g current density, there will be a phenomenon of capacity increase in the later period. The greater the current density, the more obvious the phenomenon of capacity increase, due to the insertion and extraction of ions during the cycle And the volume change of the Sn-based active material makes the interlayer distance of MXenes further increase, releasing additional storage space for lithium ions.

Figure 5. STCT composite electrochemical performance test: (a) CV curve (0.1mV/s), (b) charge-discharge curve at 500mA/g, (c) rate test, (d) rate of related pillar MXenes Performance comparison, (ef) cycle performance at 500 mA/g and 2000 mA/g.
Because MXenes itself is a capacitive material, it is necessary to analyze the capacitance ratio. In the CV curves of different sweep speeds, as the sweep speed increases, the polarization increases. The fitting analysis of logi and logv shows that the slope Greater than 0.5, between 0.5 and 1, the contribution of capacitance cannot be ignored. The high lithium storage capacity of the STCT composite material contains a relatively high proportion of capacitance contribution, and it increases with the increase of the scanning speed, so it has excellent rate performance, and the composite material itself is bonded with a stable microstructure to make it have Good cycle stability.

Figure 6. STCT composite electrochemical performance test: (a) CV curve at different sweep speeds, (b) logi and logv relationship curves, (c) capacitance contribution at 0.1mV/s sweep speed, (d) at different sweep speeds Capacitance contribution ratio diagram.
Source: Nano Express
This information comes from the Internet for academic exchange only. If there is any infringement, please contact us to delete
- Previous: 探索Nb4C3Tx MXene:单层厚度下的
- Next: Advanced Functional Ma


 mxene academic
mxene academic