 Bacterial infection is the most common problem encountered in the process of wound healing/skin regeneration. Severe inflammation significantly increases the number of diseases caused by wound infection and reduces the quality of wound healing. In addition, the treatment of chronic wounds caused by bacterial infections is a significant burden on the medical system and patients. The main clinical treatment strategy for infectious wounds is the use of antibiotics. However, due to drug resistance and the lack of a suitable wound repair microenvironment, the therapeutic effects of anti-infection and wound healing are not ideal. Therefore, it is very necessary to develop a multi-functional treatment strategy that can resist multidrug-resistant bacterial infections and promote infectious wound healing/skin regeneration.
Bacterial infection is the most common problem encountered in the process of wound healing/skin regeneration. Severe inflammation significantly increases the number of diseases caused by wound infection and reduces the quality of wound healing. In addition, the treatment of chronic wounds caused by bacterial infections is a significant burden on the medical system and patients. The main clinical treatment strategy for infectious wounds is the use of antibiotics. However, due to drug resistance and the lack of a suitable wound repair microenvironment, the therapeutic effects of anti-infection and wound healing are not ideal. Therefore, it is very necessary to develop a multi-functional treatment strategy that can resist multidrug-resistant bacterial infections and promote infectious wound healing/skin regeneration.
Recently, the team of Professor Zhang Qiuyu of Northwestern Polytechnical University designed a self-healing, conductive and tissue-adhesive 2D Ti3C2Tx MXene-based scaffold (HPEM), which has strong hemostatic ability, good biocompatibility and promotes MRSA infection The ability of wound healing. The preparation, multifunctional properties and biomedical applications of HPEM scaffolds have been comprehensively evaluated in vitro and in vivo. Related work was published on "ACS Nano" with the title "Conductive Antibacterial Hemostatic Multifunctional Scaffolds Based on Ti3C2Tx MXene Nanosheets for Promoting Multidrug-Resistant Bacteria-Infected Wound Healing".

[Design and synthesis of HPEM scaffold] First, prepare Ti3C2Tx MXene nanosheets (MXene@PDA) coated with oxidized hyaluronic acid (HCHO) and polydopamine, and combine low molecular weight polyethyleneimine (PEI) with Glycerol triacrylate (GTA) is reacted to produce branched PGE polymers. Then, the HPEM scaffold was prepared by Schiff base reaction between the amino group of PGE, the aldehyde group of HCHO and the ketone carbonyl group of MXene@PDA.
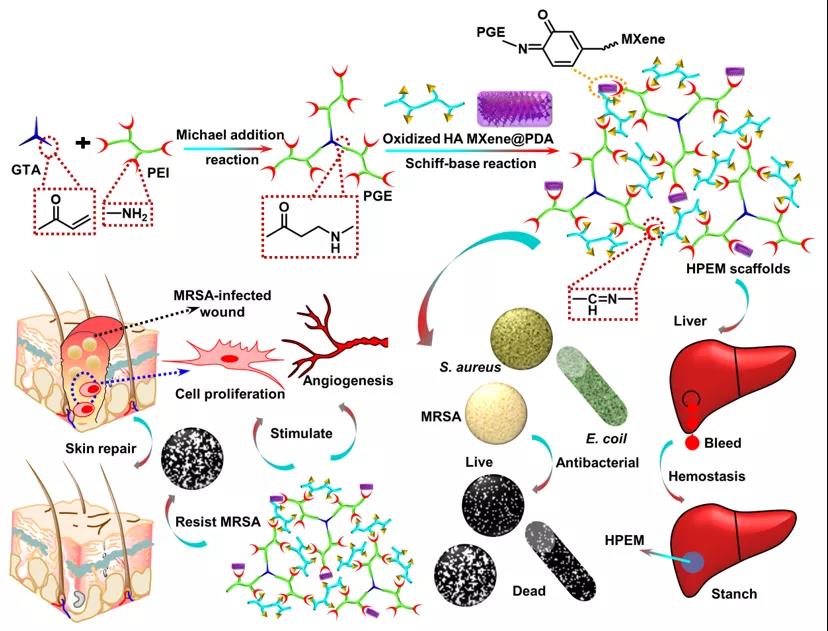
Preparation of HPEM scaffold and its application in wound healing caused by multi-drug resistant bacteria
The 1H NMR, FTIR, SEM, TEM and EDS energy spectra were used to characterize the structural characteristics of the raw materials and the HPEM scaffold, which proved the successful preparation of the porous HPEM scaffold.
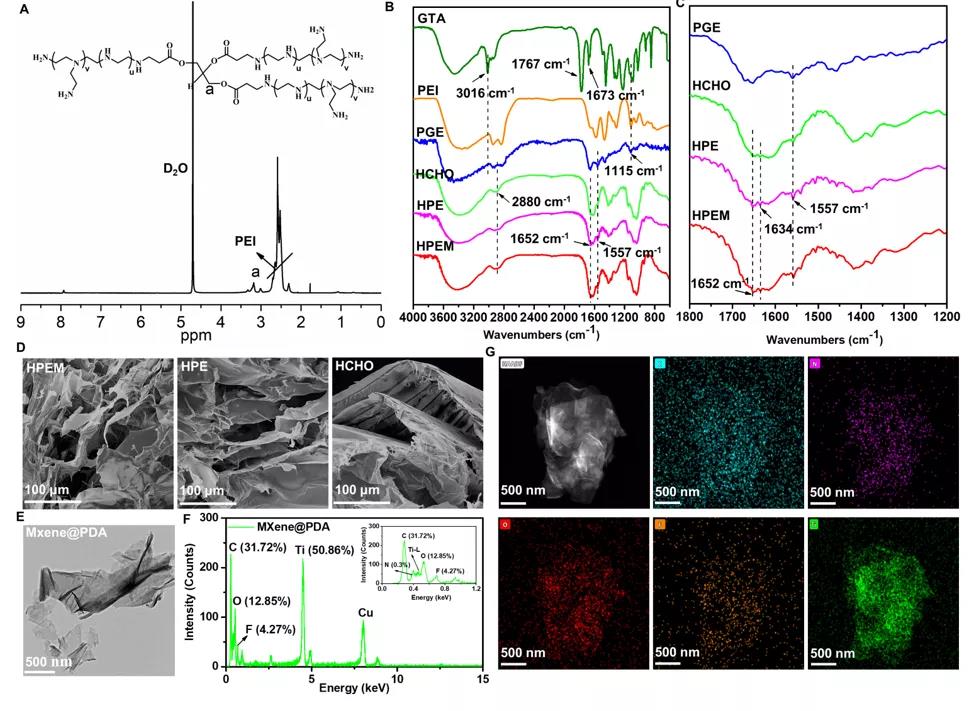
Structural characterization of the scaffold
[Characterization of multifunctional properties] Researchers have characterized the rheological properties, self-healing ability, electrical conductivity and adhesion ability of the HPEM scaffold. Even at 4oC, the storage modulus of HPEM is still higher than that of the control HPE, indicating that MXene@PDA participates in the formation of the HPEM support network. The shear thinning effect and self-healing properties of the HPEM scaffold were evaluated by measuring the G and G under the shear strain of 1% to 1000%, and it was confirmed that the HPEM scaffold was prepared by Schiff base reaction rather than simply Mix and form. In addition, the HPEM scaffold cut into two pieces can heal quickly after being placed for 2 minutes, which further proves that the HPEM scaffold has good self-healing properties due to the dynamic Schiff base bonds in the network. The HPEM scaffold can still maintain the gel state after being placed at room temperature for 24 hours, indicating its good stability. With the extension of the storage time, the dynamic Schiff base bonds in the network begin to break. After 36 hours of standing, some liquid can be observed in the stent. After 48 hours, HPEM became a solution state due to the complete rupture of the Schiff base bond in the scaffold network. In addition, due to the high conductivity of MXene@PDA, the conductivity of HPEM (2.89±0.025 S/m) is much higher than that of HPE (0.69±0.027 S/m). At the same time, the normal stress of HPEM on fresh pigskin is the highest in all test groups, indicating that it has better tissue adhesion. The above results indicate that, due to its better self-healing ability, skin adhesion ability and higher electrical conductivity, HPEM scaffold has great potential as a multifunctional dressing for the repair of infectious skin wounds.
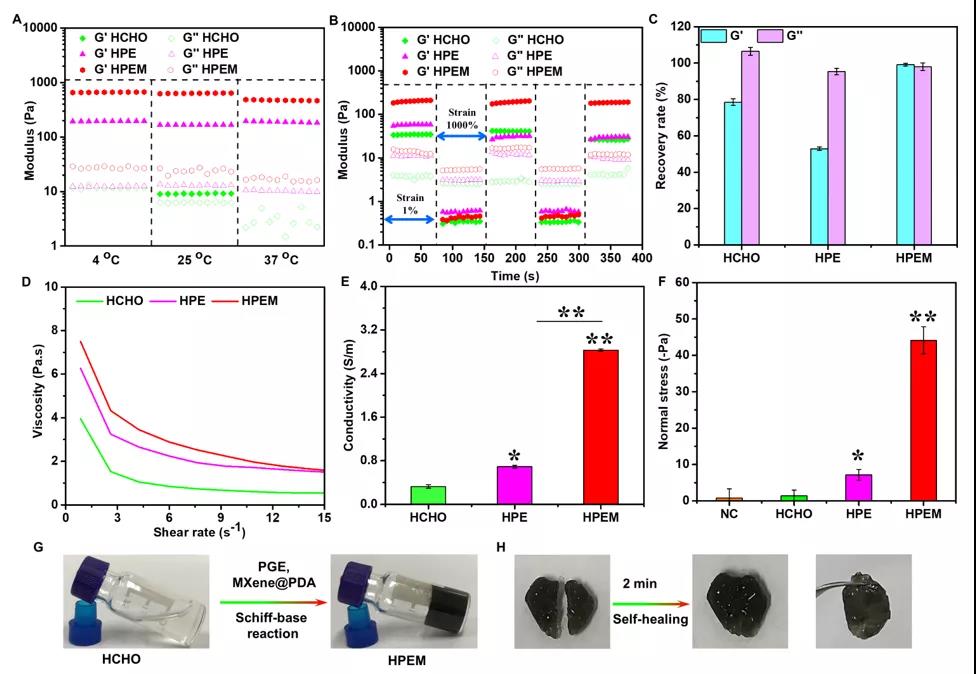
Evaluation of the multifunctional characteristics of the stent
[Antibacterial activity and hemostatic ability] Use the bacterial counting method to determine the antibacterial activity of the HPEM scaffold against Escherichia coli (Gram-negative bacteria), Staphylococcus aureus (Gram-positive bacteria) and Methicillin-resistant Staphylococcus aureus (MRSA) active. The HPEM scaffold can effectively inhibit the growth of more than 98.6% of Escherichia coli and 99.9% of Staphylococcus aureus, indicating that it has good antibacterial activity. Moreover, the HPEM stent can also effectively inhibit MRSA that is resistant to many commonly used antibiotics. Its antibacterial efficiency (99.03%) against MRSA is equivalent to vancomycin (98.9%) and much higher than that of ampicillin (44.8%). ). A mouse hemorrhagic liver model and tail bleeding time measurement method were used to evaluate the hemostatic ability of the HPEM stent. After 5 s of treatment with the HPEM stent, the bleeding at the bleeding site was quickly controlled. After 60 s, the blood loss of the control group was about 155.6±7.63 mg. The blood loss in the HPEM group was only 24.4±5.39 mg. In addition, compared with the control group, the coagulation time of the HPEM group was significantly reduced, indicating that HPEM has a good hemostatic ability. Due to its strong anti-infection and hemostatic ability, HPEM is expected to be a multifunctional scaffold that can promote the healing of infectious wounds.
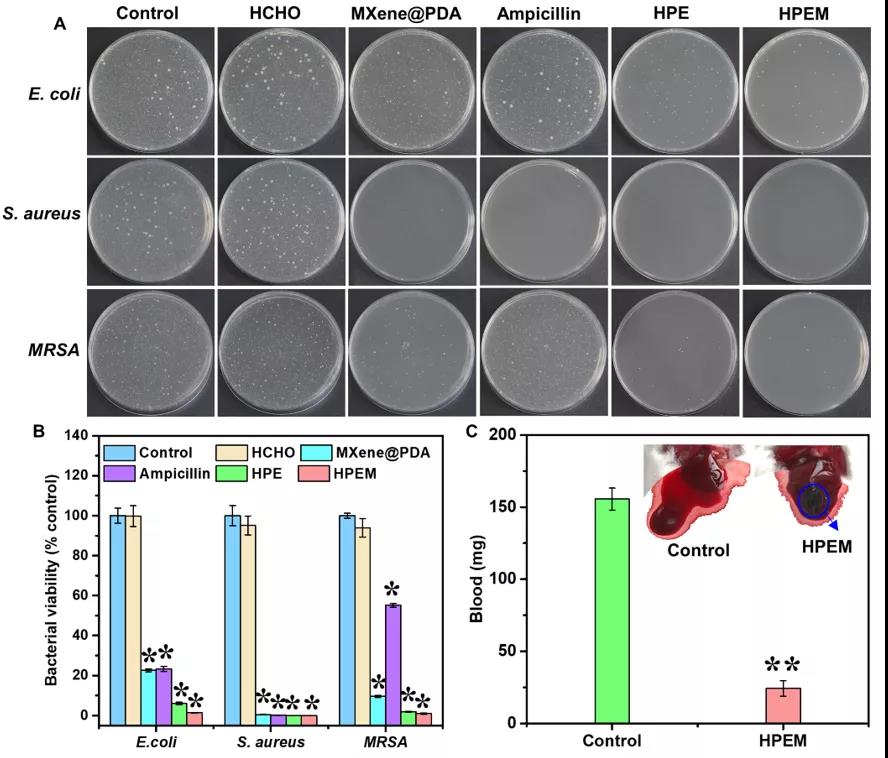
Determination of antibacterial activity and hemostatic ability
[Toxicity, cell proliferation and gene expression determination] To evaluate the cytotoxicity of HPEM and the control group at a concentration of 5-25μg/mL in L929 cells. The results showed that even at a concentration of 25μg/mL, after 24 hours of treatment of L929 cells, HPEM and controls (except PGE) showed higher cell viability (over 99%), indicating that the HPEM scaffold has good biocompatibility Sex. The cell proliferation results showed that all groups showed many viable cells (green) after co-incubation at a concentration of 5μg/mL for 1 day. Subsequently, as the number of viable cells increased significantly after 3 and 5 days of co-incubation, rapid cell proliferation was observed in all groups. After a total of 5 days of incubation, the Alamar Blue kit was used to quantitatively analyze the cell proliferation. The fluorescence intensity of the HPEM group was the highest, which further proved the promoting effect of HPEM on cell proliferation. The possible reason is that due to its excellent electrical conductivity, HPEM can build a cellular communication network by enhancing electrical transmission, thereby up-regulating the expression of related genes to promote cell proliferation. Smooth muscle α-actin (α-actin) plays a vital role in wound healing because it can promote the proliferation and differentiation of fibroblasts. In addition, type III collagen (COL III) and vascular endothelial growth factor (VEGF) are very important for granulation tissue reorganization, basement membrane regeneration, vascular endothelial cell migration and angiogenesis during wound healing, respectively. The gene expression of α-actin in the HPEM group was significantly higher than that in the control group, indicating that HPEM can promote cell proliferation by increasing the expression of α-actin. In addition, among the groups, the HPEM group had the highest mRNA levels of COL III and VEGF. These results indicate that HPEM may promote wound healing by up-regulating the gene expression of α-actin, COL III and VEGF.
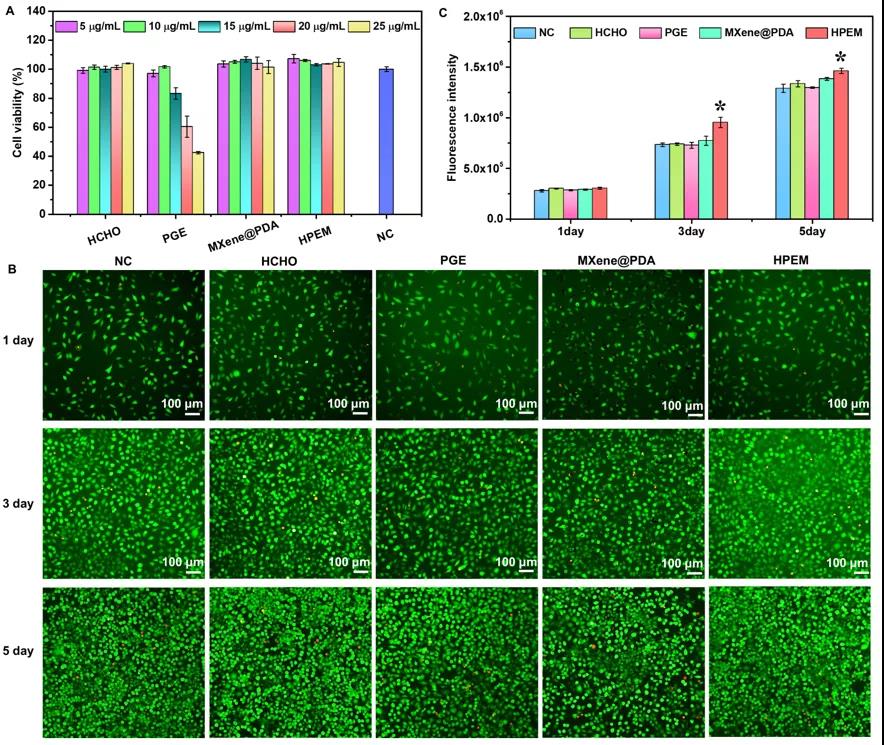
Evaluation of cytotoxicity and cell proliferation of scaffolds
【MRSA Infection Wound Healing Evaluation】Using the full-thickness skin wound model of MRSA infection to evaluate the healing ability of HPEM scaffolds on infectious skin wounds. On the 3rd day after surgery, the wound size in the HPEM group was smaller than that in the other groups. At the same time, due to bacterial infection, some yellow pus exudation was observed in the 3M group. On the 7th day after the operation, the wound area of the HPEM group was significantly reduced, while the wound area of the HPE, 3M and blank groups were still as high as 66.2%, 88.7% and 62.8%, respectively. On the 14th day after surgery, the infectious wound in the HPEM group was almost completely healed (the wound closure rate was 96.31%), and the wound site was basically covered by new skin tissue. In contrast, the wounds in the blank group and the 3M group were covered by eschar, while the HPE group healed slightly faster than the two control groups. The above results indicate that the HPEM scaffold can promote wound healing in MRSA infection. In order to evaluate the antibacterial ability of the HPEM scaffold in vivo, the bacteria on the wound tissue on the 3-14th day after the operation were cultured on LB agar plates. In addition, F4/80 immunofluorescence staining and Ly6G immunohistochemical analysis were performed on macrophages and neutrophils locally recruited due to bacterial infections to evaluate the severity of MRSA infection in each group on the third day after surgery . The above results indicate that the HPEM scaffold can effectively resist infection caused by MRSA in the body and reduce the inflammatory response, and therefore can accelerate wound healing under bacterial infection. H&E staining and Masson trichrome staining were used to analyze the histological morphology of wound samples during healing. The results show that the HPEM scaffold can promote the healing process of infectious wounds by accelerating the formation of granulation tissue and increasing the deposition of collagen. The immunohistochemical analysis of Ki67 and the immunofluorescence staining of α-smooth muscle actin (α-SMA) and CD31 were used to study the cell proliferation, angiogenesis and angiogenesis of wound tissue on the 7th day after surgery. The above results indicate that the HPEM scaffold can effectively resist MRSA infection, promote granulation tissue formation, collagen deposition, cell proliferation, vascular endothelial differentiation and angiogenesis, and ultimately significantly accelerate the wound healing process of MRSA infection.
Anti-infection and MRSA wound healing in the body
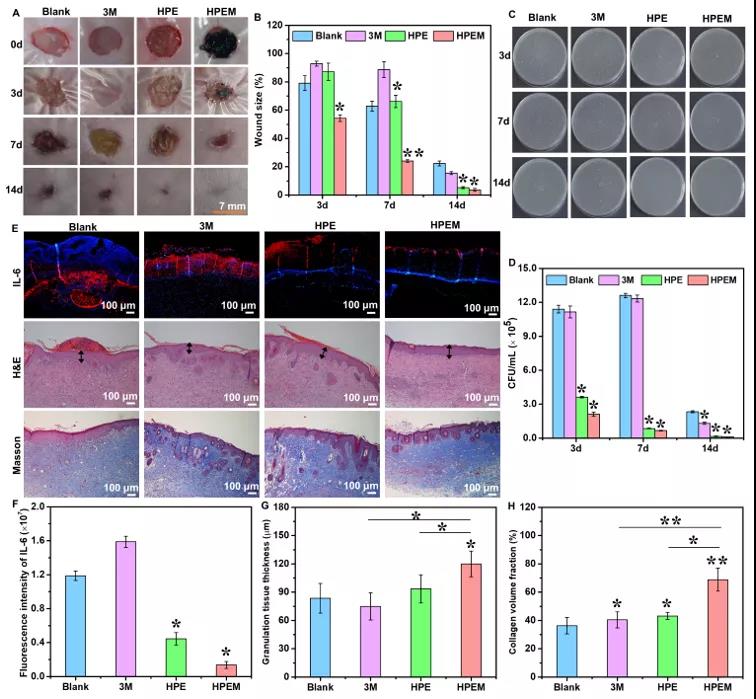
Immunohistochemistry and immunofluorescence staining analysis of wound healing assay
Summary In conclusion, researchers have developed a biodegradable conductive multifunctional 2D Ti3C2TxMXene-based scaffold (HPEM) for wound healing in MRSA infections. The optimized combination of HCHO, PGE and MXene@PDA endows HPEM with multifunctional properties such as excellent rheology, self-healing ability, electrical conductivity and tissue adhesion properties. In addition, the HPEM scaffold exhibits high antibacterial activity against E. coli, Staphylococcus aureus and MRSA, can effectively stop bleeding and promote cell proliferation, and has negligible in vitro toxicity. In addition, HPEM can significantly enhance the wound healing of MRSA infection by anti-infection, promote cell proliferation, angiogenesis, up-regulate α-SMA and CD31, promote granulation tissue formation, collagen deposition, vascular endothelial differentiation and angiogenesis. This study shows that 2D MXene@PDA nanosheets play a vital role in the healing process of infectious wounds. This work shows that the multifunctional conductive HPEM scaffold can promote early angiogenesis of infectious wounds and accelerate wound healing and skin reconstruction.











