已传文件:photo/1631586161.png

1. Article overview
Although the research and development of Ti3C2Tx electrodes for high-performance batteries and supercapacitors has made continuous progress, the role of negative ions in electrochemical energy storage and their ability to insert MXene sheets under positive voltage has not yet been elucidated. Ten years after the discovery of MXene electrodes, information about the possibility of inserting anions in stacked MXene electrodes is still being questioned. Because Tis anodic oxidation severely limits its stable range of positive potential in diluted aqueous solutions, the possibility of anion insertion in concentrated aqueous solutions and aprotic electrolytes has also been evaluated. In order to solve this problem, the author performed in-situ gravimetric electrochemical quartz crystal microbalance measurement with dissipation monitoring in high-concentration LiCl and LiBr electrolytes, which makes the working range of the MXene electrode significantly positive. Extension. In addition, halogen is one of the smallest anions and should be easier to intercalate between the MXene layers than polyatomic anions. Based on the mass change in the positive voltage range and complementary density functional theory calculations, it is proved that MXene is unlikely to insert anionic species in the potential range of interest for capacitive energy storage. This can be explained by the strong negative charge on the functional group-terminated Ti3C2Tx flakes.
Two, graphic guide
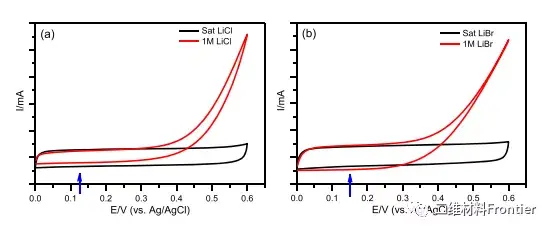
Figure 1. MXene electrode measured cyclic voltammogram at 10 mV/s in diluted and concentrated LiCl (a) and LiBr (b) electrolyte solutions. The blue arrows indicate the OCV of the electrodes in the centralized system.
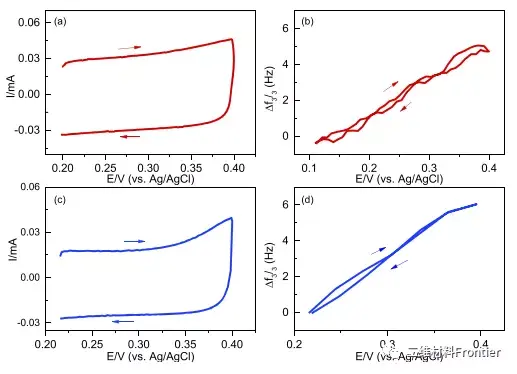
Figure 2. The MXene electrode is cycled for the first time in concentrated LiCl (a) and LiBr (c) electrolyte solutions at 20 mV/s. The corresponding frequency changes measured in LiCl and LiBr are shown in (b) and (d), respectively. Arrows indicate the scan direction and related frequency changes.
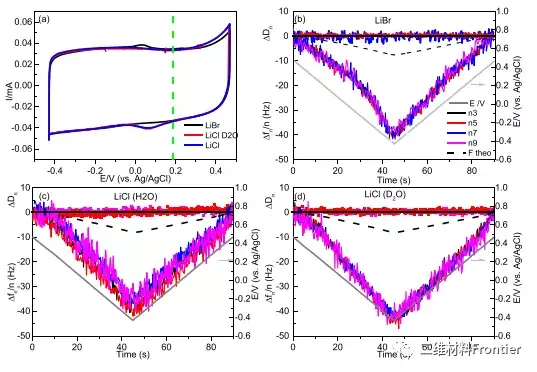
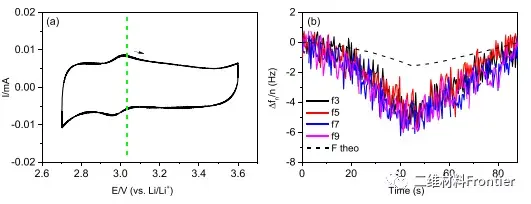
Figure 3. (a) Cyclic voltammogram of MXene electrode dissolved in concentrated LiCl in H2O and D2O and in LiBr electrolyte solution at 20 mV/s. (b d) The changes in frequency and dissipation of the third to ninth overtones of saturated LiBr, LiCl and LiCl in D2O are (b) (d), respectively. The theoretical frequency change is indicated by the black dashed line.
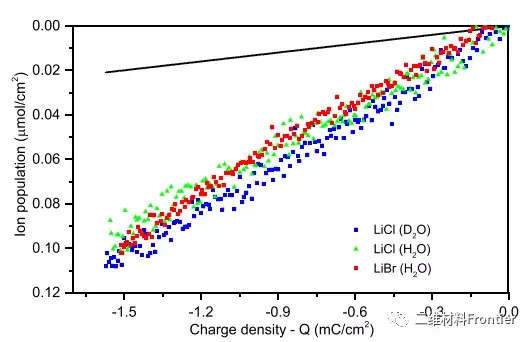
Figure 4. The ion density of LiBr, LiCl, and LiCl varies with charge density. The change in ion population calculated by Faradays equation is represented by a black solid line.
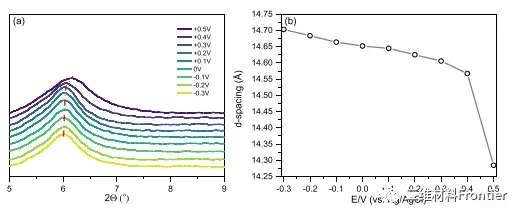
Figure 5. (a) The change of the measured 2θ value between 0.3 and 0.5 V during the potential sweep. (b) Corresponding changes in the distance between MXene electrodes d.
Figure 6. (a) CV response in EC:DMC of Ti3C2 electrode circulated in 1 M LiPF6. The black arrow indicates the scanning direction. (b) Frequency change recorded during potential sweep. The theoretical frequency change is shown as a black dashed line.
The experimental and theoretical studies provide evidence that MXenes cannot electrochemically insert anions within the reported voltage range of 0.3 to +0.5 V. This discovery reveals the previously published XRD and dilation studies conducted by Lin et al. 3 and Jackel et al. Shows the contraction of the MXene layer under the action of a positive potential, rather than the expected expansion. According to the authors observation, the electronegative surface terminator of the MXene layer prohibits anion insertion, and this shrinkage may be caused by further extraction of spontaneously inserted/adsorbed cations. Since MXenes can effectively carry larger cations and molecules, such as Cs+ and tetramethylammonium, with a radius equal to or larger than that of Cl− or Br−, it seems that electrostatic repulsion is the main reason for repelling anions. This is based on the theory The calculation is supported. The current research results indicate that the surface engineering of the MXene sheet to form a positively charged terminal may allow negative ions to be reversibly inserted into the MXene electrode. On the other hand, Ti3C2Tx can be used as a cation exchange membrane for water treatment, battery separators and capacitor deionization electrodes.
Article link:
https://pubs.acs.org/doi/10.1021/jacs.1c03840.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately



 1. Article overview
1. Article overview