已传文件:photo/1766544328.png
The reduction dehydroaromatization reaction of nitrogen-containing aromatic compounds can generate three-dimensional heterocyclic compounds of significant value, which are widely present in natural products, drugs, and agricultural chemicals. The traditional pyridine dehydroaromatization reaction has poor selectivity and can generate various dihydropyridine isomers. Among the various dihydropyridines, 1,4-dihydropyridine is particularly important. Many drugs contain this structural unit, and it can serve as a multifunctional reagent and synthetic intermediate in numerous organic transformation reactions. Therefore, the selective reduction of pyridine to 1,4-dihydropyridine is an important and challenging topic.
Due to the aromaticity of pyridine and quinoline, they cannot be directly reduced by mild reducing agents. Usually, this process requires first activating them as pyridinium salts to promote the addition of hydride. In terms of regioselectivity, the nucleophilic addition of hydride is more likely to generate 1,2-isomers rather than 1,4-isomers. The reasons are as follows: (1) The C2 position has stronger electrophilicity than the C4 position because it is closer to the nitrogen atom; (2) 1,2-dihydropyridine and 1,2-dihydroquinoline are thermodynamically more stable than the corresponding 1,4-isomers, which is due to the larger conjugated structure of 1,2-isomers. To achieve 1,4-selectivity, a Lewis acid with high steric hindrance as a catalyst or stoichiometric reagent is needed to activate pyridine by forming a pyridinium salt, but this strategy is ineffective for substrates with electron-withdrawing groups (EWG) at the C2 position.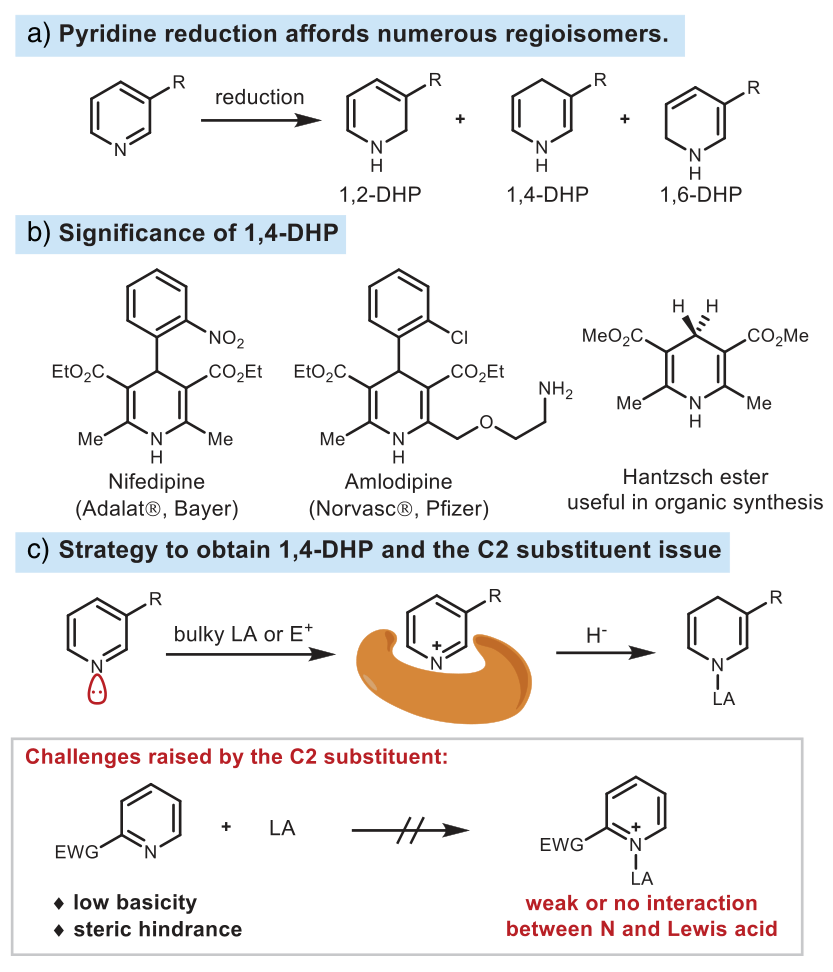
Figure 1. Research background
Inspired by the report on the separation of double oxo carbines (carbon double positive ions) by the Bertrand team (Nature 2023, 623, 66, click to read details), the Liu Wenbo research group at Sun Yat-sen University envisioned that these carbon double positive ions have sufficient strong electrophilicity to activate pyridine, and their flexible structure provides the possibility to regulate the steric hindrance at the C2 site. The authors synthesized various carbon double positive ions with different steric hindrances using commercially available reagents and finally obtained the target product with a 75% yield using DMI (1,3-dimethyl-2-imidazolone) as the optimal activating reagent and NMe3・BH3 as the source of hydride. Other common activating reagents could only obtain the target product with a 15% nuclear magnetic resonance yield and low selectivity (C4:C6 = 1.5:1) (Figure 2).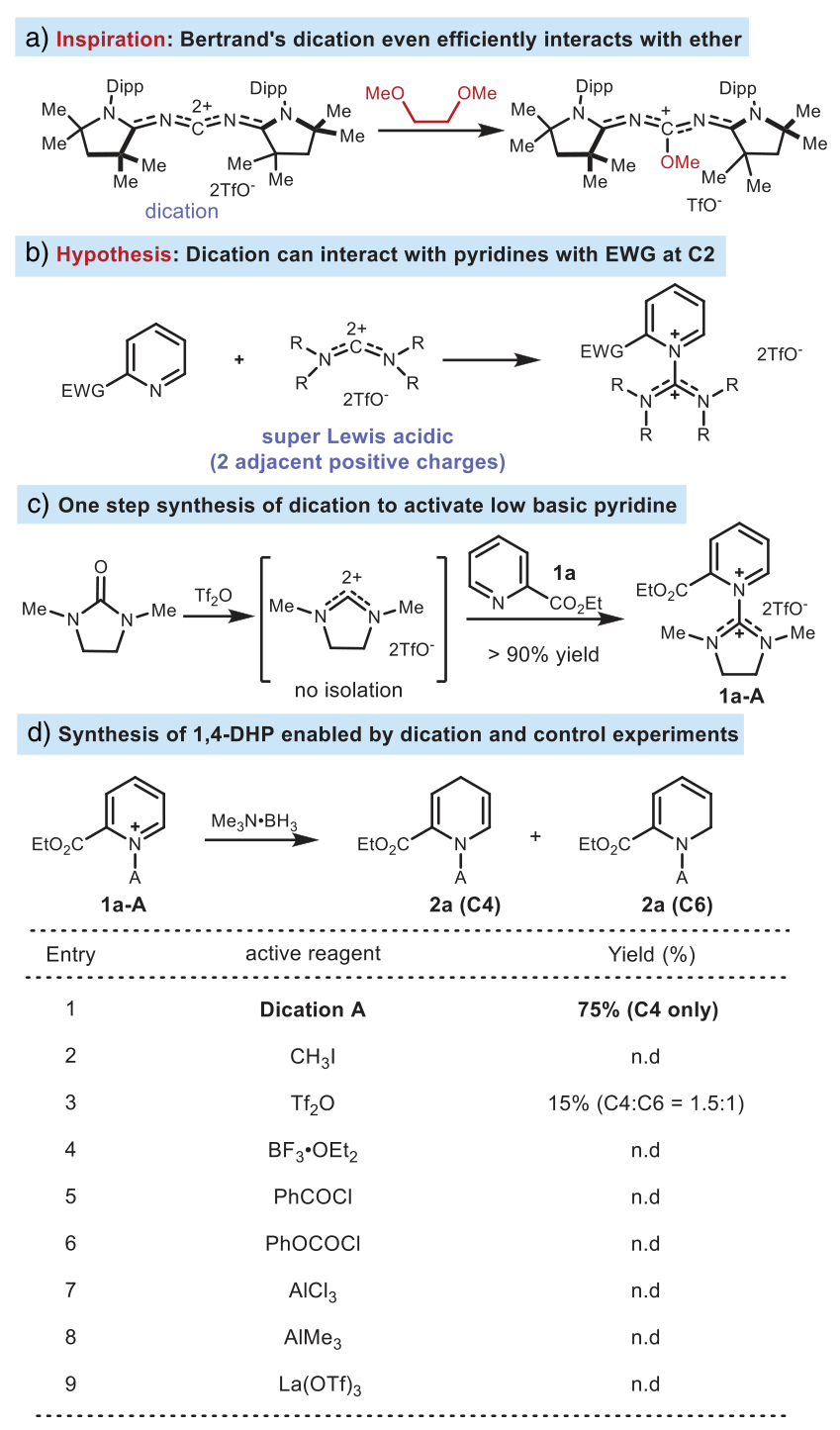
Figure 2. Research and design of large steric hindrance activating reagents
Under the optimal conditions, the authors studied the compatibility of the substrates, as shown in Figure 3. Various C2-substituted pyridines (containing -CN, ester group, -F, -Cl, -Br) could be efficiently reduced to the corresponding 1,4-dihydropyridine (2a-2e, yield 54%-75%), which was the first report of the conversion of such C2-substituted pyridines to 1,4-dihydropyridine. Quinoline compounds also successfully underwent 1,4-reduction reactions, achieving good results (2j-2m, yield 56%-70%). In addition to C2-substituted pyridines and quinolines, various C3-substituted pyridines were also applicable, including aryl-substituted pyridines (2n, 83%; 2y, 77%) and various functional groups-substituted pyridines, such as -I (2ab, 82%), -Br (2o, 80%), -Cl (2p, 76%), -F (2q, 74%), -CN (2r, 62%), -CF3 (2s, 81%), ester group (2t, 72%), -OPh (2aa, 82%), conjugated ester (2u, 57%), and borate ester (2z, 73%). Surprisingly, even the pyridine with C4 substitution also produced 1,4-dihydropyridine (2ae, 80%; 2af, 42%), rather than 1,2-dihydropyridine. This indicates that the carbon double positive ion reagent has a strong 1,4-directing effect. To further evaluate the substrate scope of this dehydroaromatization scheme, the authors placed several complex molecules containing pyridine and quinoline structures under standard reaction conditions, and all yielded products with moderate to high yields (2ag-2al, 51%-87%). Notably, in all the above cases, only 1,4-selectivity was observed.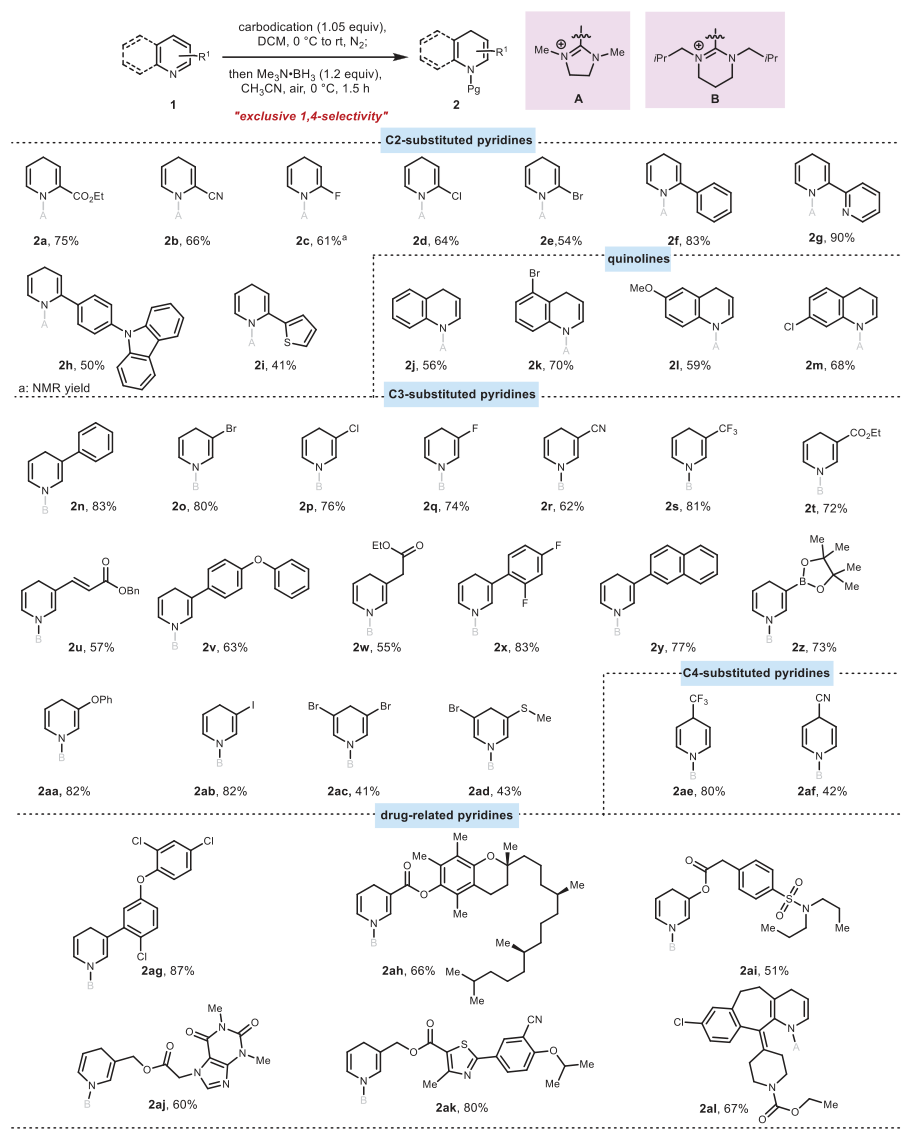
Figure 3. Substrate Extension
In organic synthesis, directly preparing 3,5-disubstituted pyridines through the C-H bond functionalization of pyridine is quite challenging. In recent years, the Wang Xiaochen research group has developed a strategy that uses borane catalysis and 1,4-dihydropyridine as an intermediate to synthesize various C3 or C5 functionalized pyridines (J. Am. Chem. Soc. 2022, 144, 4810, click for details). However, oxidative electrophilic reagents such as N-bromosuccinimide (NBS) and bromine would cause 1,4-dihydropyridine to re-aromatize instead of brominating the alkenes. Using this strategy proposed by the authors, efficient bromination of 1,4-dihydropyridine can be achieved, and then the brominated 1,4-dihydropyridine can be oxidized to obtain C5-brominated pyridine. In addition, the authors also used carbon nucleophilic reagents to prepare 4,5-functionalized pyridine derivatives. Under copper catalysis conditions, the C5 position trifluoromethylation was achieved using 1,4-dihydropyridine as the intermediate (Figure 4 bottom).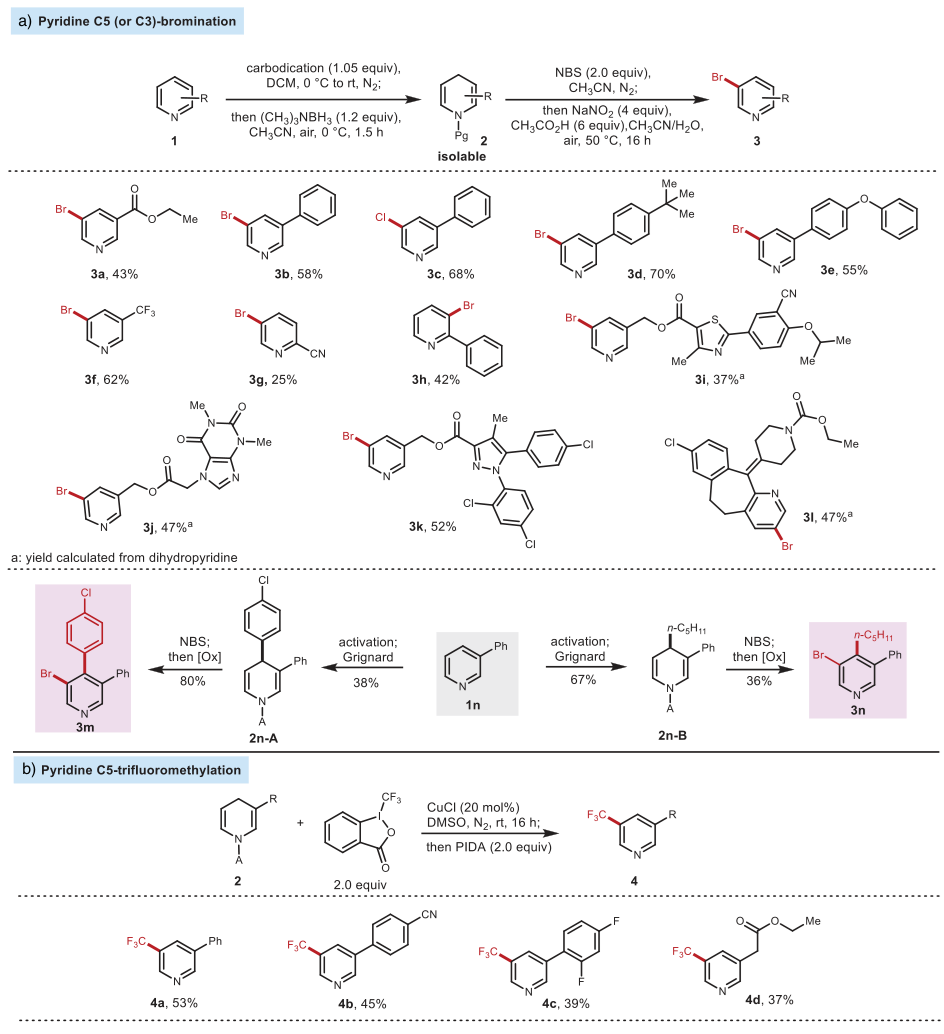
Figure 4. Synthetic Applications
Finally, to demonstrate the practicality of this method in deuterium labeling, the authors prepared representative deuterium-labeled pyridines. Using D2O as the deuterium source, 1,4-dihydropyridine undergoes hydrogen/deuterium exchange under the presence of acid, and then is oxidized to synthesize C5-deuterated pyridine (see Figure 5). Using NaBD4 as a reducing agent, C4-deuterated pyridine can be obtained.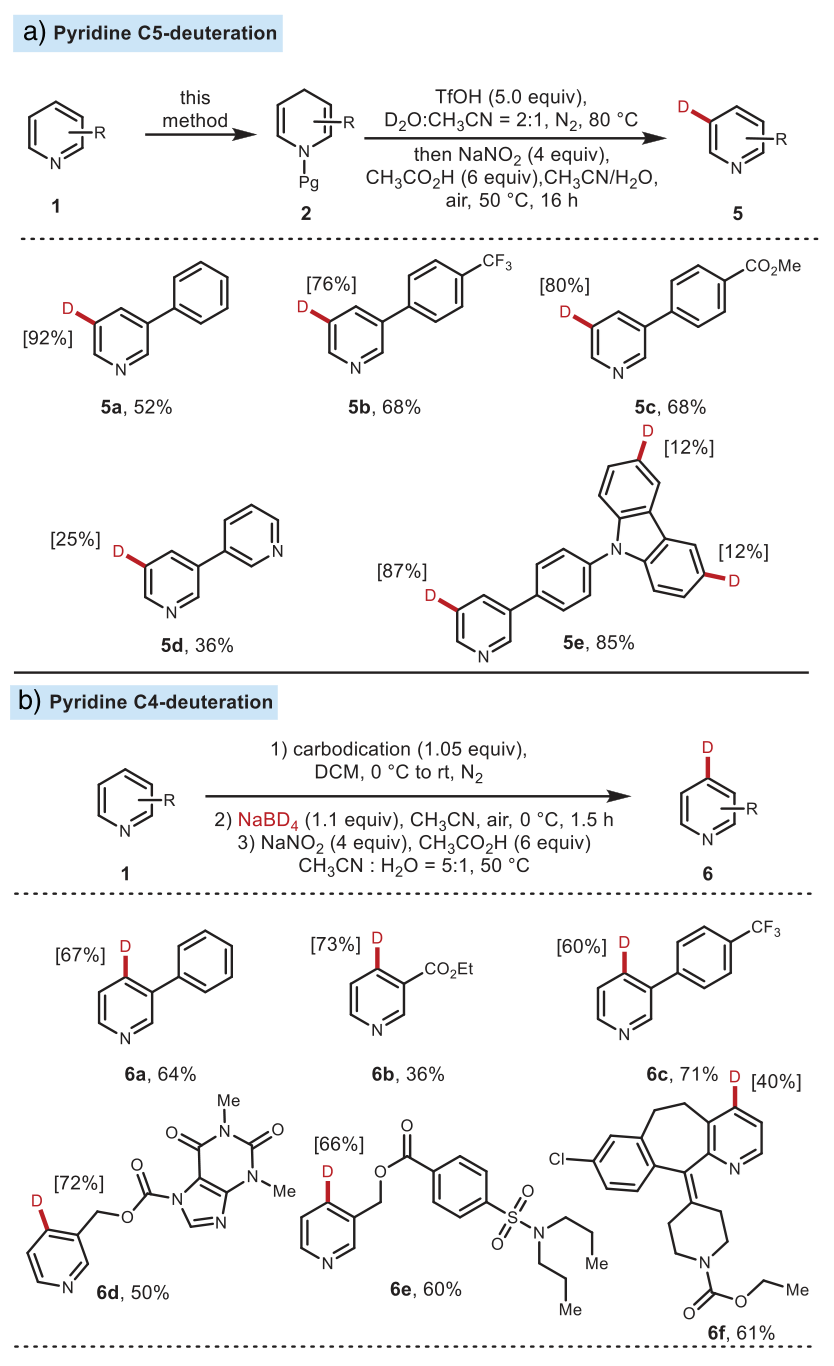
Figure 5. Synthesis of Deuterated Pyridines from 1,4-Dihydropyridine Summary
The research group led by Liu Wenbo from Sun Yat-sen University proposed a pyridine dealkylation strategy using carbon double positive ions as the activating reagent, which can specifically generate 1,4-dihydropyridine. For the first time, a pyridine with an electron-withdrawing group at the C2 position was selectively reduced with good yield to 1,4-dihydropyridine. Due to the high reactivity of the carbon double positive ion-pyridine adduct, mild reducing agents (such as NMe3∙BH3 and NaBH4) can be used instead of strong reducing agents (LiAlH4 or LiBHEt3), and it is compatible with various functional groups. Using 1,4-dihydropyridine as a stable dealkylation intermediate, C5-brominated, C5-trifluoromethylated, C5-deuterated, and C4-deuterated pyridines were successfully prepared, fully demonstrating the synthetic application value of this scheme. Given the ease of obtaining the starting materials and reagents, the simplicity of the reaction conditions, and the importance of 1,4-dihydropyridine, it is expected that this scheme will have broad application prospects. This work was supported by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, and Sun Yat-sen University. The related work was published in Angewandte Chemie International Edition. Professor Liu Wenbo from the School of Chemistry of Sun Yat-sen University is the corresponding author, the School of Chemistry of Sun Yat-sen University is the corresponding unit, and 2023-level master student Huang Xiaofeng is the first author of the paper.








