Achilles’ heel of sulfide electrolyte-based all-solid-state lithium batteries: interface problems
QQ Academic Group: 1092348845
Detailed
Recently, the team of Nancewen of Tsinghua University and the team of Yutao Li from the University of Texas at Austin published a review titled "Interfacial challenges for all-solid-state batteries based on sulfide solid electrolytes" in the well-known domestic journal Journal of Materiomics.
The first author of the paper: Tsinghua University doctoral student Wang Shuo. Corresponding author: South policy paper and Academician Dr. LI Yu-tao. Sulfide solid electrolytes (such as thiophosphate electrolytes) have the highest room temperature ion conductivity (~10-2 S cm-1) among solid lithium ion conductors, so all-solid-state batteries with high energy density and safety are increasing People pay more attention. However, the problem of the interface between the sulfide electrolyte and the electrode hinders its practical application in all solid-state lithium batteries. During the charge and discharge cycle, the interface is unstable due to the side effects of sulfide and the electrode, poor solid-solid contact, and lithium dendrite problems. This paper analyzes the interface problems existing in all solid-state batteries based on sulfide electrolytes, and focuses on the strategies to solve these interface problems and stabilize the electrode-electrolyte interface. At the same time, the development prospects of all-solid-state battery interface engineering based on sulfide electrolyte are prospected. Content highlights
1. This review system summarizes the interface problems of all-solid-state lithium batteries based on sulfide electrolytes and the corresponding solutions. The interface problems mainly include the physical contact failure of the electrode/electrolyte, the side reactions of the positive electrode/electrolyte, carbon/electrolyte, lithium metal/electrolyte, and lithium dendrites. Improving the ionic conductivity of the positive active material, preparing nano-scale active materials, coating the surface of the active material, and preparing artificial SEI at the Li/sulfide interface can effectively solve the above interface problems.
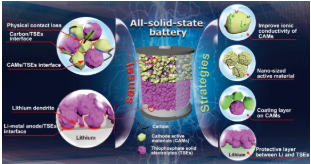
2. Physical contact failure usually occurs at the positive active material/sulfide electrolyte interface, and the lithium metal negative electrode/sulfide electrolyte interface. This is caused by the volume expansion of the positive active material and the growth of lithium dendrites during the lithium deposition process. Corresponding mitigation strategies include (1) applying constant external pressure during battery cycling, (2) preparing nano-scale composite cathodes by ball milling, (3) selecting appropriate cathode materials, such as (3) having a specific orientation, (4) ) Or zero stress/strain

3. The adverse interaction between the electrode/sulfide electrolyte interface in all solid-state lithium-ion batteries includes (a) the space charge layer formed by the chemical potential difference between the positive electrode active material and the sulfide electrolyte; (b) the inter-element Interdiffusion (such as the Co element in LCO and the P element in LPS will interdiffusion during cycling), and (c) the chemical reaction between the intercalated cathode material and the sulfide electrolyte to form the cathode/electrolyte intermediate phase. (D) Some common suppression strategies include doping oxygen in the sulfide electrolyte, coating the surface of the electrode material (such as LiNbO3 coating on the LCO surface) and doping modification of the cathode material, such as Al The element-doped LCO forms an Al-rich LCO on the surface layer, thereby stabilizing the LCO/sulfide electrolyte interface.

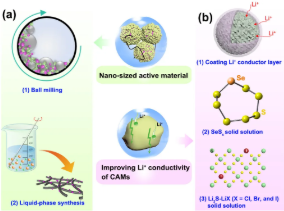
4. Strategies to reduce the interface resistance between the converted electrode material and the sulfide electrolyte include: (a) preparing nano-active electrode materials by ball milling or liquid phase method; (b) increasing the ionic conductivity of active materials, such as preparing SeSx, Li2S-LiX ( X=Cl, Br, I) solid solution, and preparation of lithium ion conductive coating on the active material.
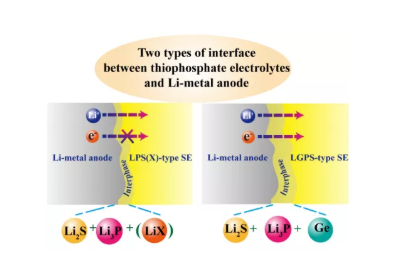
5. Two lithium anode/sulfide electrolyte interfaces in all solid-state batteries. For LPS (X) electrolyte, it will decompose into Li2S, Li3P and (LiX) after contact with metallic lithium, forming an electronically insulated ion conductive interface. The LGPS electrolyte decomposes into Li2S, Li3P and Ge after contacting with metallic lithium, forming a mixed conductor interface.
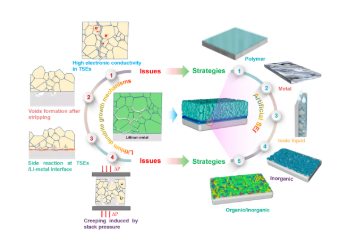
6. The nucleation and growth mechanism of lithium dendrites in the sulfide electrolyte, including the high conductivity of the sulfide electrolyte, the formation of voids in the Li negative electrode during the peeling/deposition process, the side reactions of the sulfide electrolyte/lithium metal interface, and cycling Li metal creep caused by external pressure during the process. The preparation of artificial SEIs such as polymers, metals, ionic liquids, inorganic, organic/inorganic materials on the surface of lithium metal helps to inhibit the growth of lithium dendrites and stabilize the interface.
Summary and outlook
This review summarized in detail the interface problems and lithium dendrite problems in all solid-state lithium batteries based on sulfide electrolytes. The large volume change of the electrode material causes the physical contact between the active material and the sulfide electrolyte to fail, resulting in capacity degradation. Due to the narrow electrochemical window, the sulfide electrolyte will undergo decomposition reactions when it comes in contact with the two electrodes (high voltage positive electrode and lithium metal negative electrode). The space charge layer, element interdiffusion and chemical reactions all occur at the oxide cathode/sulfide electrolyte interface, resulting in an increase in interface resistance. The electronic and ionic conductivity of S/Li2S is poor, resulting in a large charge transfer resistance between the conversion electrode material and the sulfide electrolyte, which further limits the reaction kinetics. The high intrinsic electronic conductivity of the sulfide electrolyte, defects (ie, grain boundaries), impurities, and the formation of mixed conductive interfaces have led to an increase in the local current density of the lithium-metal interface, and the formation of holes after peeling at high current densities. Each factor has a great contribution to the growth of lithium dendrites. Next, discuss methods to solve these problems. The ideal sulfide electrolyte should have high ionic conductivity, wide electrochemical stability window and good (electro) chemical stability, so that it can be used in conjunction with high voltage/large capacity positive electrode and lithium metal negative electrode to achieve high energy density/power Density all solid state battery. Low electronic conductivity is a necessary condition for inhibiting the growth of lithium dendrites; however, this is still challenging, and only a few interface strategies can be used to solve the sulfide electrolyte/electrode interface problem. In order to develop new high-performance sulfide materials and overcome this problem, it is necessary to conduct more research on the inherent characteristics of sulfide electrolyte materials. The development of a positive electrode material with low strain and high capacity can help reduce the physical contact failure between the positive electrode active material and the sulfide electrolyte, which is usually caused by the volume expansion of the electrode material during cycling. The preparation of nano-active materials can also help stabilize the cathode/sulfide interface by reducing charge transfer resistance and improving electrochemical reaction kinetics. In addition, increasing the ionic conductivity of the positive electrode composite material helps to increase the utilization rate of the active material. In addition to constructing good ion and electron conduction paths, the positive electrode composite material should also be strong enough to compensate for the volume expansion of the electrode material without sacrificing performance. For this reason, the content and type of conductive carbon in the positive electrode composite should be optimized to minimize the decomposition of sulfide, thereby reducing the impedance of the battery and improving the rate performance of the battery. The use of an electronically insulating and ion-conducting coating on the surface of the oxide positive electrode active material is beneficial to inhibit the side reaction between the oxide positive electrode and the sulfide electrolyte. These coatings need to be further studied to improve their ionic conductivity, reduce their electronic conductivity, maintain sufficient elasticity to reduce the volume change of the electrode material, and provide chemical stability of the high-voltage-resistant cathode material. In addition, these high-performance coatings can reduce the interface resistance between the oxide cathode and the sulfide electrolyte, thereby improving the rate performance of the all-solid-state lithium battery. The artificial SEI layer prepared on the surface of the sulfide electrolyte or lithium negative electrode can inhibit the interface reaction, thereby inhibiting the growth of lithium dendrites. Although LiF is a good artificial SEI, its ionic conductivity is very low. In order to achieve uniform lithium exfoliation/deposition at high current densities, it is necessary to further explore the ability to prepare thin SEIs with high ionic conductivity, low electronic conductivity and high specific surface energy. External pressure is a good external parameter to adjust the performance of all solid-state lithium batteries, but there are also some shortcomings that must be vigilant. External pressure can ensure good interface contact to inhibit the formation of voids during the lithium stripping process, and inhibit the contact loss of the positive electrode interface due to the volume change of the active electrode material, but the creep of the lithium metal must be controlled to prevent it from reaching over High levels, which short-circuit the battery. In order to accelerate the commercialization of all-solid-state batteries, the use of bulky battery cycle external pressure systems should be avoided. Literature information
Shuo Wang, Ruyi Fang, Yutao Li*, Yuan Liu, Chengzhou Xin, Felix H. Richter, Ce-Wen Nan* Journal of Materiomics https://doi.org/10.1016/j.jmat.2020.09.003
Information source: Frontier of Lithium Battery
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
- Previous: Hydrogel, another Scie
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier
