Sargent Nature Energy, express delivery of achievements such as Wan Lijun, Meng Ying, Zhang Xianzheng, Sun Xueliang, Zhi Chunyi 20201210
QQ Academic Group: 1092348845
Detailed
1. Nature Energy: Amine capture solution for efficient electrochemical CO2 reduction
Carbon dioxide capture technology based on chemical adsorption has the potential to reduce the net emission of carbon dioxide into the atmosphere. The use of renewable energy to upgrade the electrochemistry of captured carbon dioxide into value-added products has the advantage of simple process. Amine solution can capture carbon dioxide, but the efficiency of directly converting RNHCOO into CO2 value-added products by electrochemical reduction is poor. It has not been reported that amine-CO2 compounds can achieve higher CO at a working current density greater than 50 mA cm-2. Conversion rate electrolyzes higher value products. In view of this, Edward H. Sargent of the University of Toronto et al. reported on the direct electrolysis of amine-CO2 into value-added products through the use of alkaline cation-specific electrochemical double layers (EDL).
The main points of this article: 1) Electrochemical impedance spectroscopy and in-situ surface enhanced Raman spectroscopy show that when alkali metal cations are introduced into the amine-CO2 electrolyte, they will change the composition of the EDL, thereby promoting heterogeneous electrons to carbamate Transfer. The H+ generated in situ through the bipolar membrane releases CO2 from the amine capture solution to achieve direct electrolysis. Direct electrolysis of MEA-CO2 provides a way to circumvent the limitation of low CO2 solubility. 2) With the aid of alkaline cations, under optimal conditions, at a current density of 50 mA cm-2, the Faraday efficiency of CO2 to CO conversion is 72%. 3) In addition, the recyclability of the amine-CO2 electrolyte is also proved. In the process of multiple capture-electrolysis cycles, the CO Faraday efficiency does not change much, and this strategy can recover the amine solvent.
Electrocatalysis Academic QQ Group: 740997841
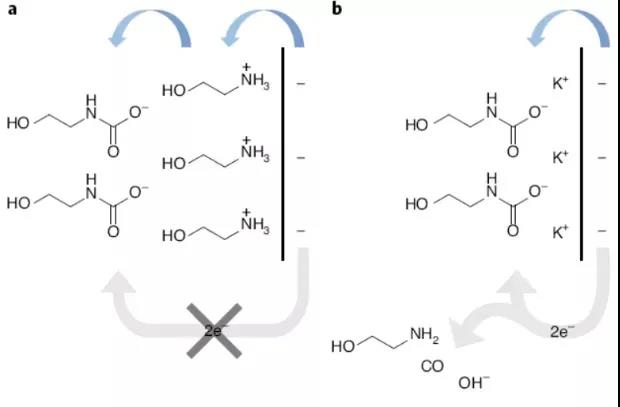
Lee, G., Li, YC, Kim, JY. et al. Electrochemical upgrade of CO2 from amine capture solution. Nat Energy (2020).DOI: 10.1038/s41560-020-00735-zhttps://doi.org/10.1038 /s41560-020-00735-z
2. Nature Catal.: Different FeNx sites of Fe-N-C materials in proton exchange membrane fuel cells
Fe-N-C materials are the most promising alternatives for catalytic oxygen reduction reactions in acid polymer fuel cells, but the problem of catalyst degradation has always been an obstacle to achieving their long-term applications. At present, there is a lack of understanding of the loss of its catalytic activity, which slows the pace of optimizing the synthesis of highly stable catalysts. In view of this, Professor Frédéric Jaouen of the University of Montpellier and others have conducted in-depth research and understanding of the structure-activity relationship of different FeNx active sites in Fe-NC materials for the subsequent preparation of high-stability Fe-NC catalysts for proton exchange membrane fuels The battery provides strong experimental evidence.
The main points of this article: 1) The study found that during the catalysis process, two different FeNx sites (S1 and S2) of the Fe-N-C catalyst changed significantly. S1 is degraded through conversion to iron oxide, while the structure and quantity of S2 are unchanged. 2) 57Fe Mossbauer spectroscopy test results show that the first two sites of the reaction both contribute to the oxygen reduction reaction activity, but only S2 plays a major role after 50 hours of reaction. 3) In situ 57Fe Mössbauer spectra in an inert gas and related theoretical calculations together show that S1 is high-spin FeN4C12, and S2 is low-spin or medium-spin FeN4C10.
Electrocatalysis Academic QQ Group: 740997841

Picture Li, J., Sougrati, MT, Zitolo, A. et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat Catal ( 2020).DOI: 10.1038/s41929-020-00545-2https://doi.org/10.1038/s41929-020-00545-2
3. Nature Catal. Review: Water-induced deactivation of cobalt-based Fischer-Tropsch synthesis catalyst
Catalyst deactivation is the main challenge faced by industrial catalytic processes and will greatly increase production costs. For the Fischer-Tropsch reaction, the water produced is usually assumed to be the culprit of catalyst deactivation. Cobalt nanoparticles may be oxidized to CoO, thereby forming a mixed metal oxide with the carrier, or sintered into larger particles. The actual occurrence and effects of the water-induced inactivation process have long been controversial in the literature. In view of this, Professor Michael Claeys of the University of Cape Town in South Africa and others discussed the feasibility of these deactivation methods in depth around the water-induced deactivation of cobalt-based Fischer-Tropsch synthesis catalysts and emphasized the importance of in-situ characterization.
The main points of this article: 1) The controversy of water-induced deactivation is mainly due to the lack of a suitable model catalyst system and the application of characterization technology. The current technology cannot directly monitor specific cobalt phases. The latest developments in in-situ characterization techniques provide valuable insights into various deactivation mechanisms in the cobalt-based Fischer-Tropsch synthesis process. In particular, the exploration of the formation of cobalt carrier compounds during synthesis, activation and catalysis can accelerate the understanding of the mechanism of water-induced deactivation. 2) For this solid state reaction, it is very necessary to fully understand and characterize the process of the oxidation of active cobalt to cobalt oxide and the formation of cobalt carrier compound from cobalt oxide. Recently, research on hydrothermal sintering and water-induced reoxidation of cobalt oxide has attracted more and more attention, and has begun to provide necessary experimental evidence for understanding these deactivation mechanisms.
Nanocatalysis academic QQ group: 256363607

Picture Wolf, M., Fischer, N. & Claeys, M. Water-induced deactivation of cobalt-based Fischer–Tropsch catalysts. Nat Catal (2020).DOI: 10.1038/s41929-020-00534 -5https://doi.org/10.1038/s41929-020-00534-5
4. Acc. Chem. Res.: Hollow carbon-based nanospheres: synthesis strategies, mechanism insights and electrochemical applications
Hollow carbon-based nanospheres (HCNs) have been proven to show good potential in many research fields, especially in electrochemical devices for energy conversion/storage. The current schemes for the synthesis of HCNs mainly rely on template-based routes (TBRs). These routes have the advantage of simple process in creating hollow structures, but face the challenges of time-consuming operation, low product yield, and serious environmental problems caused by hazardous etchants. At the same time, they have shown insufficient capabilities in constructing complex carbon-related structures. Therefore, it is very urgent to develop innovative strategies for HCNs that do not require additional template agents. This not only ensures precise control of the key structural parameters of HCNs with specified functions, but also is an environmentally friendly and scalable method suitable for their practical applications.
In view of this, academician Wan Lijun and researcher Cao Anmin of the Institute of Chemistry of the Chinese Academy of Sciences and others reviewed the latest research progress in the development of a template-free strategy for the creation of HCN, with emphasis on obtaining hollow space without involving additional templates. Mechanism understanding of structure.
The main points of this article: 1) It is proved that carbon-based particles themselves can be used as a general platform to create hollow structures by effectively adjusting their internal chemical composition. Through reaction control, the precursor particles are synthesized into solid particles with well-designed inhomogeneities in the form of different chemical parameters, such as molecular weight, crystallinity and chemical reactivity, thereby not only creating hollow structure particles inside , Also has the ability to adjust key characteristics, including composition, porosity and size structure. Therefore, the function of the prepared HCN can be systematically adjusted or optimized for its application background. More importantly, the synthetic methods discussed are simple and environmentally friendly methods with the potential to scale up production. 2) The nano-engineering technology of HNCs is of special importance for its application in various electrochemical energy storage and conversion systems. In these systems, charge transfer and structural stability are an important issue. Therefore, the potential of HCN in battery systems such as sodium ion batteries (NIB) and potassium ion batteries (KIB) is particularly emphasized. 3) Studies have shown that precise control of the structure of HCN is essential to provide sufficient reaction sites, excellent charge and mass transfer kinetics, and optimal design of elastic electrode structures, and it also provides a model system suitable for studying complex metal ions Storage mechanism, such as storing Na+ in a hard carbon anode.
In short, this work is expected to promote the development of HCNs in a variety of applications such as energy conversion and storage, catalysis, biomedicine, and adsorption.
Picture De-Shan Bin et al. Manipulating Particle Chemistry for Hollow Carbon-based Nanospheres: Synthesis Strategies, Mechanistic Insights, and Electrochemical Applications. Acc. Chem. Res., 2020.DOI: 10.1021/acs.accounts.0c00613https://doi .org/10.1021/acs.accounts.0c00613
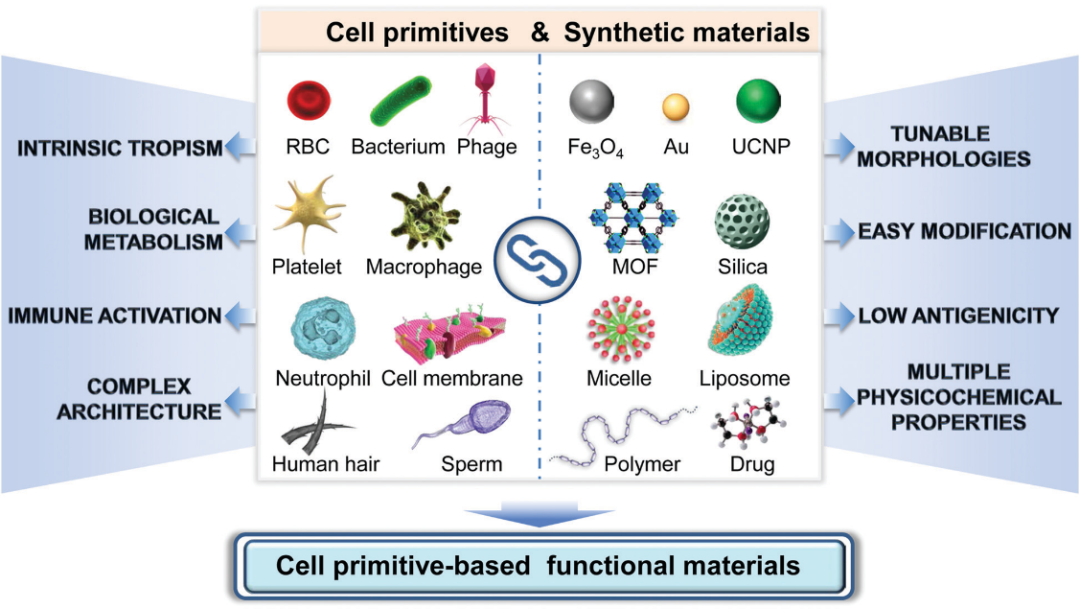
5. Chem. Soc. Rev.: Cell primordium biomimetic functional materials for enhancing cancer treatment
Professor Zhang Xianzheng from Wuhan University reviewed the research on cell primordium biomimetic functional materials in enhancing cancer treatment. The main points of this article: 1) Cell primordium functional materials combine the advantages of natural materials and nanotechnology, and have become an attractive cancer treatment drug. The cytoplasma has unique biological functions, such as long-term internal circulation, tumor-specific targeting and immune regulation. In addition, synthetic nanomaterials with unique physical and chemical properties have also been widely used as drug carriers or anti-cancer agents for the treatment of cancer. Therefore, the combination of these two materials is expected to produce biomaterials with multiple functions and high biocompatibility, which can then achieve precise cancer treatment. 2) This article summarizes the research progress of materials with tumor treatment functions based on cell primordia in recent years; introduces different cell protozoa, including bacteria, phages, cells, cell membranes and other biologically active substances. Activity and function; At the same time, the author also discussed the strategy of combining it with synthetic materials, especially nanosystems to construct functionalized biomaterials. Finally, the author also looked forward to future research directions in this field.
Biomimetic Materials Academic QQ Group: 111658060
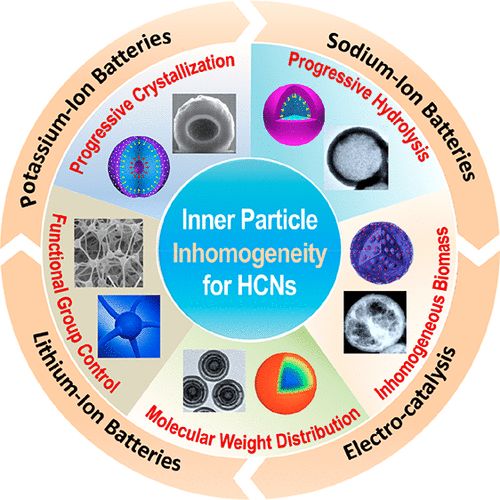
Picture Guo-Feng Luo. et al. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chemical Society Reviews. 2020DOI: 10.1039/d0cs00152j https://pubs.rsc.org/en/content/ articlelanding/2020/cs/d0cs00152j#!divAbstract
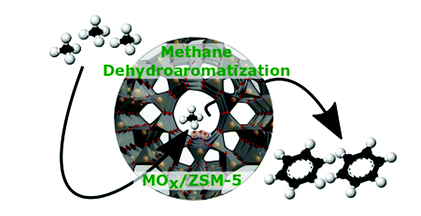
6. Chem. Soc. Rev. review: ZSM-5 supported transition metal center for methane activation
Benzene is an important organic intermediate used in the synthesis of chemicals such as ethylbenzene, cumene, cyclohexane, nitrobenzene and alkylbenzene. Among many gas-liquid conversion processes, methane anaerobic dehydrogenation aromatization (MDA) has a good application prospect because it avoids the deep oxidation of CH4 to CO2 and does not require co-reactants. The metal oxide supported by ZSM-5 is an important class of multifunctional catalysts. Because of the various reactions it catalyzes, it has always attracted the attention of researchers in academia and industry.
Recently, Israel E. Wachs of Lehigh University in the United States reviewed the use of ZSM-5 supported transition metal oxide catalysts (MOX/ZSM-5, M=V, Cr, Mo, W, Re, Fe) in methane dehydrogenation and aromatization. The latest research progress in benzene production.
Key points of this article: 1) The author focused on the basic, in-situ and operando spectroscopic studies of MOX/ZSM-5 catalyst. In particular, it summarizes the anchor sites of the supported oxygen species on the ZSM-5 carrier, the molecular structure of the MOX sites on the initial dispersed surface, the nature of the active sites during the MDA reaction, the reaction mechanism, the rate control steps, and the dynamics of the MDA reaction Learning and catalyst activity. 2) The author finally pointed out the basic problems to be solved in the future MOX/ZSM-5 catalyst for MDA research, and put forward his personal opinions.
Nanocatalysis Academic QQ Group: 256363607
Picture Daniyal Kiani, et al, Methane activation by ZSM-5-supported transition metal centers, Chem. Soc. Rev., 2020DOI: 10.1039/d0cs01016bhttps://doi.org/10.1039/D0CS01016B
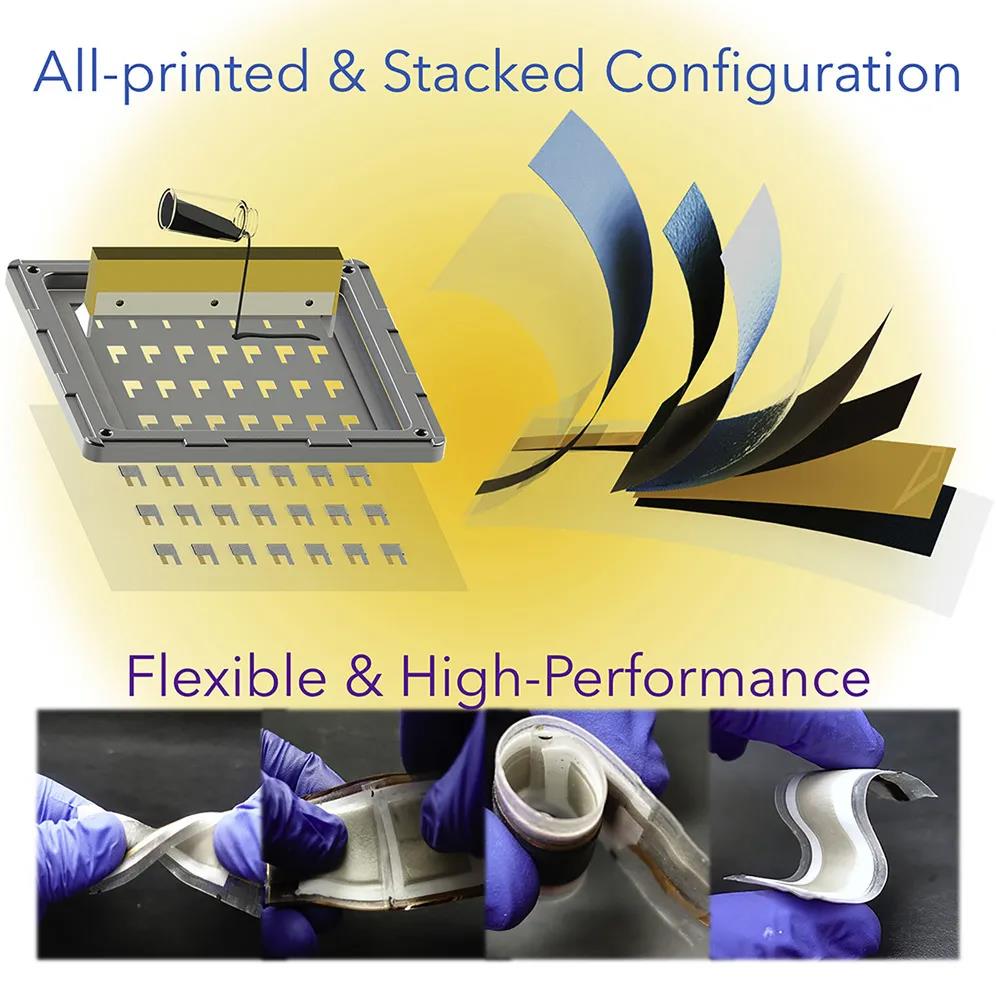
7. Joule: High-performance fully printed rechargeable AgO-Zn battery for flexible electronics
A cost-effective and scalable battery with good mechanical and electrochemical properties is essential for the wide application of flexible electronic products. Recently, Professor Ying Shirley Meng of the University of California, San Diego, Joseph Wang reported on a new manufacturing process for all-printed, flexible and rechargeable AgO-Zn batteries. The developed AgO-Zn battery has ultra-high area capacity, low impedance and good rechargeability, and is a practical energy storage solution for flexible electronic products.
The main points of this article: 1) The manufacture of AgO-Zn batteries uses low-cost, high-throughput, layer-by-layer printing formula powder-elastomer composite ink to form current collectors, zinc anodes, AgO cathodes and their corresponding separators. Using low-occupancy stacking structure, potassium hydroxide (KOH)-polyvinyl alcohol (PV
A) The hydrogel acts as a low impedance electrolyte sandwiched between two fully printed electrodes. In addition, based on a thermoplastic styrene-ethylbutylene-styrene block copolymer (SEBS) elastomer substrate, the assembled battery can be directly heated and vacuum sealed to preserve the electrolyte and ensure proper battery pressurization. This manufacturing and assembly process can be applied to different battery sizes with adjustable surface capacity, and the battery shape can be customized for specific applications. 2) By making full use of the higher oxidation state of AgO, the AgO-Zn battery can have a higher areal capacity at more than 54 mAh/cm2, while maintaining a low internal resistance (10 Ω) in various applications. In addition, with the optimized cycle configuration, the AgO-Zn battery can be charged more than 80 times, maintain a 0.2-1 C discharge, and maintain a low impedance during each cycle without significant capacity loss. In addition, the manufactured battery exhibits excellent durability against repeated bending and distortion. 3) The AgO-Zn battery successfully supplied power to a flexible display system equipped with an integrated microcontroller (MCU) and a Bluetooth module that requires high-current pulse discharge, demonstrating its performance in powering typical flexible electronic products.
The all-printed AgO-Zn battery provides a practical solution for various electronic products, which is of great significance for the future development of high-performance flexible batteries.
Battery Academic QQ Group: 924176072
Yin et al., High Performance Printed AgO-Zn Rechargeable Battery for Flexible Electronics, Joule (2020) DOI: 10.1016/j.joule.2020.11.008https://doi.org/10.1016/j.joule.2020.11.008
8. Joule review: Design and manufacturing of 3D printed batteries
3D printed batteries are a unique type of energy storage device with outstanding features of micron size and diversity of appearance, which are essential for the miniaturization and customization of electronic products. Therefore, clarifying the 3D printing design in battery materials and architecture is essential to optimize the performance and customization of 3D printed batteries. In view of this, Professor Chen Yunfei and Zhiyang Lyu of Southeast University reviewed the latest research progress of 3D printing batteries, including the relationship between printable materials and printing technology and reasonable design.
The main points of this article: 1) The author first outlines the unique features of 3D printing technology, and then focuses on summarizing printable battery modules and the general methods for making them printable. 2) The author next clarified the important role of 3D printing design in the module architecture, battery configuration and effective solutions that hinder battery performance. 3) The author finally proposed further research directions for 3D printing batteries in terms of functional materials, advanced printing technology and new device design.
3D printing academic QQ group: 247384403
Lyu et al., Design and Manufacture of 3D-Printed Batteries, Joule (2020) DOI: 10.1016/j.joule.2020.11.010https://doi.org/10.1016/j.joule.2020.11.010
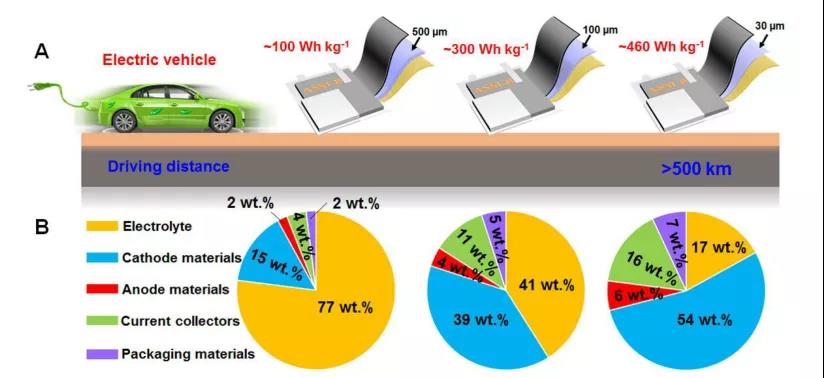
9. EES review: the latest research progress in thin electrolytes for high-energy density solid-state lithium batteries
Solid-state lithium batteries (SSLBs) are a promising next-generation energy storage device due to their high energy density and higher safety. Solid electrolyte (SSE) is a key component of SSLBs, and its performance and physical parameters have an important influence on the electrochemical performance and energy density of the battery. In recent years, although thick SSE has been widely used in SSLB, it has disadvantages such as higher internal resistance, additional inactive material content, lower actual energy density, and higher battery manufacturing cost. Therefore, reducing the thickness of SSEs and developing high-performance thin SSEs are critical to the commercialization of SSLBS. Recently, Professor Sun Xueliang from the University of Western Ontario in Canada reviewed the latest research progress in the manufacture of thin SSE and its application in SSLBs.
The main points of this article: 1) The author first outlines the different methods and manufacturing processes of thin SSE films in SSLB. Then summarized the reasonable design of thin SSE and its application in different SSLBs such as Li-ion, Li-S and Li-O2 batteries. 2) The author clarified the advanced characterization techniques used to reveal the Li+ transport mechanism in SSE, the structure/component evolution of SSE at the interface and its effect on ion conductivity and battery performance. Then, the weight/volume energy density of SSLB pouch batteries with SSE thickness less than 100 μm was evaluated. 3) The author finally clarified the combination of other key parameters and thin SSE to achieve the goal of the actual weight/volume energy density of SSLBs greater than 300 Wh kg-1/500 Wh L-1, and looked forward to the future development of the thin SSE in SSLBs direction.
Battery Academic QQ Group: 924176072
Xiaofei Yang, et al, Recent Advances and Perspectives on Thin Electrolytes for High-Energy-Density Solid-State Lithium Batteries, Energy Environ. Sci., 2020DOI: 10.1039/D0EE02714Fhttps://doi.org/10.1039/D0EE02714F
10. EES: Cum+nPb100SbmTe100Se2m (CLAST) with a layered structure to achieve higher thermoelectric performance
Thermoelectric technology can use the Seebeck effect to directly convert thermal energy into electrical energy and is a promising energy source. Among high-performance thermoelectric materials, lead telluride (PbTe) is particularly prominent in the intermediate temperature range. Recently, p-type PbTe achieved record-breaking ZT and ZTave by controlling the valence band convergence and the collaborative optimization strategy of nanostructure. Although p-type PbTe has shown high thermoelectric properties, the thermoelectric properties of n-type PbTe still remain at a relatively low level, which hinders the wide application of PbTe-based materials. Moreover, previous research work cannot achieve both high power factor and low lattice thermal conductivity. Therefore, it is of great significance to develop new strategies to achieve the high thermoelectric performance of PbTe-based materials by balancing the transport characteristics of carriers and phonons.
Recently, Professor Lidong Zhao from Beijing University of Aeronautics and Astronautics, and Researcher Gao Xiang from Beijing High Voltage Research Center (HPSTAR) reported a high-performance n-type Cum+nPb100SbmTe100Se2m (CLAST) thermoelectric material, which has a hierarchical structure including copper-based nanoprecipitation and atomic gaps. .
The main points of this article: 1) The results show that the alloying of a small amount of CuSbSe2 (about 3%) in CLAST can make the room temperature carrier concentration as high as 1.7×1018 cm-3, and then optimize the power factor, and at the same time precipitate the embedded Cu from the matrix Base nanostructures to reduce the thermal conductivity of the lattice. In addition, adding extra Cu atoms to CLAST can form gaps, further increasing the carrier concentration to 3.0×1018 cm-3, and the room temperature carrier mobility reaches 1227.8 cm-1 s-1, so that Cu3.3Pb100Sb3Te100Se6 The power factor reaches 20.0 μWcm-1K-2. In addition, at 623 K, the Cu gap and a large amount of Cu-based nano-precipitates can strongly scatter a large number of phonons, which reduces the lattice thermal conductivity in Cu3.3Pb100Sb3Te100Se6 to 0.44 Wm-1K-1. 2) In the CLAST sample The copper-based layered structure can synergistically optimize the transport characteristics of phonons and carriers, and the ZT at 300 K and 723 K are respectively 0.5 and 1.4. In the temperature range of 300-723 K, the ZT value of Cu3.3Pb100Sb3Te100Se6 is as high as 0.94, which is better than other high-performance n-type PbTe-based thermoelectric materials.
Thermoelectric Materials Academic QQ Group: 699166559
Siqi Wang, et al, Hierarchical structures lead to high thermoelectric performance in Cum+nPb100SbmTe100Se2m(CLAST), Energy Environ. Sci., 2020DOI: 10.1039/D0EE03459Bhttps://doi.org/10.1039/D0EE03459B
11. EES: Activate I0/I+ redox in the I2-Zn water-based battery to achieve high voltage
Rechargeable iodine battery has complete electron transfer and abundant valence, and has broad application prospects in portable energy storage. However, compared with the standard hydrogen electrode (SHE), at a potential of 0.54 V, the reaction is limited to a single redox reaction of I-/I0, resulting in a low voltage of 1.30 V when zinc is used as the negative electrode. Recently, Professor Chunyi Zhi from City University of Hong Kong reported that in the F- or Cl-rich electrolyte, the I-terminal halogenated Ti3C2I2 Mxene positive electrode and zinc foil negative electrode were used to achieve iodine (I-/ I0/I+) multivalent redox chemistry.
The main points of this article: 1) The study found that the Ti3C2I2//Zn battery based on the optimized 2 M ZnCl2 + 1 M KCl electrolyte has a two-step reaction: in addition to the conventional I-1/I0 conversion at 1.30 V, it is also I0/I + redox occurred, which greatly improved the electrochemical performance. Unsurprisingly, the higher-order voltage increases the battery capacity and energy density to 205% and 330%, respectively. 2) Due to the efficient electronic conduction and confinement of the Ti3C2I2 Mxene intermediate layer, the reaction kinetics is significantly improved and the shuttle effect is effectively suppressed. Therefore, the battery has a good cycle life exceeding 2800, and the capacity retention rate reaches 80%. It also has excellent rate performance (0.5 A g-1, 207 mAh g-1Ti3C2I2 and 5 A g-1 126 mAh g-1Ti3C2I2). 3) In-situ Raman spectroscopy analysis shows that the strong interaction between free Cl- ions and I+ ions in the electrolyte under high voltage is the decisive factor for activating and stabilizing I0/I+ on reversible redox.
This research work provides new insights for the design of advanced I2-metal batteries based on the new I-/I0/I+ chemistry to achieve high voltage and high capacity.
Battery Academic QQ Group: 924176072

Xinliang Li, et al, Activate I0/I+ redox in an aqueous I2-Zn battery to achieve high voltage plateau, Energy Environ. Sci., 2020DOI: 10.1039/D0EE03086Dhttps://doi.org/10.1039/D0EE03086D
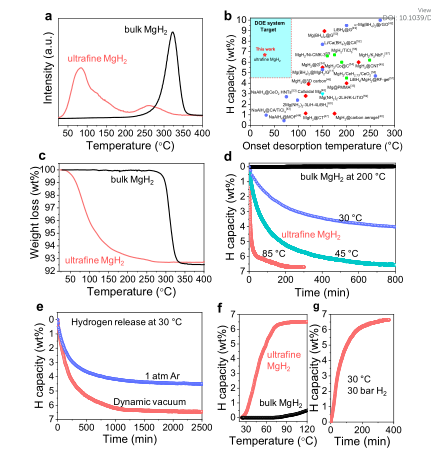
12. EES: Ultrafine MgH2 realizes 6.7wt% reversible hydrogen storage at room temperature
Magnesium hydride (MgH2) has attracted much attention in the field of hydrogen storage due to its high theoretical hydrogen storage capacity of 7.6 wt% and abundant Mg reserves. However, MgH2 has a high initial hydrogen release temperature (over 300 ℃), which is impractical. Recently, Professor Liu Yongfeng from Zhejiang University reported on the reversible hydrogen storage of unconstrained ultrafine MgH2 nanoparticles at room temperature.
The main points of this article: 1) Researchers use the characteristics of the large difference in solubility of metal hydrides and chlorides in tetrahydrofuran (THF), and propose a new ultrasonic-driven liquid-solid phase metathesis process to replace the ball milling process for the synthesis of nanoscale MgH2. In the THF reaction medium, the disproportionation reaction of MgCl2 and LiH is a liquid-solid process, which provides direct protection for the newly formed MgH2. Therefore, without a scaffold or carrier, ultrafine MgH2 nanoparticles of about 4-5nm were successfully obtained. 2) Experimental results show that thanks to the thermodynamic instability of ultrafine MgH2 and the reduction of the kinetic energy barrier, the reversible hydrogen storage of MgH2 at 30 °C reaches 6.7 wt%. In addition, compared with bulk MgH2, ultrafine MgH2 nanoparticles cycle 50 times at 150 °C, showing stable and rapid hydrogen absorption performance.
Picture Xin Zhang, et al, Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydride, Energy Environ. Sci., 2020 DOI: 10.1039/D0EE03160Ghttps://doi.org/10.1039/D0EE03160G
Information source: Nanoman
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
- Previous: Impact factor 27+, the
- Next: 1


 About us
About us
