Adv. Mater.: Self-healing/high stretch/environment-resistant/adhesive full range hydrogel electrolyte
QQ Academic Group: 1092348845
Detailed

1. Article overview
Flexible energy storage devices are at the forefront of next-generation power supplies, and one of the most important components is gel electrolyte. However, for the currently developed hydrogel electrolyte, there are more or less disadvantages. In this paper, cotton is used as raw material, tetraethyl orthosilicate is used as cross-linking agent, and glycerin is used as antifreeze. A simple and economical method is developed to construct a full range of hydrogel electrolyte. The obtained hydrogel electrolyte has high ionic conductivity, excellent mechanical properties (such as high tensile strength and elasticity), ultra-low freezing point, good self-healing ability, high adhesion and good heat resistance. It is worth noting that compared with previously reported electrolytes for aqueous zinc-ion batteries, this hydrogel electrolyte can provide a record high ionic conductivity of 19.4mS/cm at -40°C. In addition, this hydrogel electrolyte can significantly inhibit zinc dendrite growth and parasitic side reactions in the temperature range of -40 to 60°C. With this hydrogel electrolyte, a flexible quasi-solid Zn-MnO2 battery was assembled, which has an excellent energy density at a temperature of −40 to 60°C. The battery also has excellent cycle durability and high durability under various harsh conditions. This work provides new opportunities for the development of hydrogel electrolytes.
Two, graphic guide
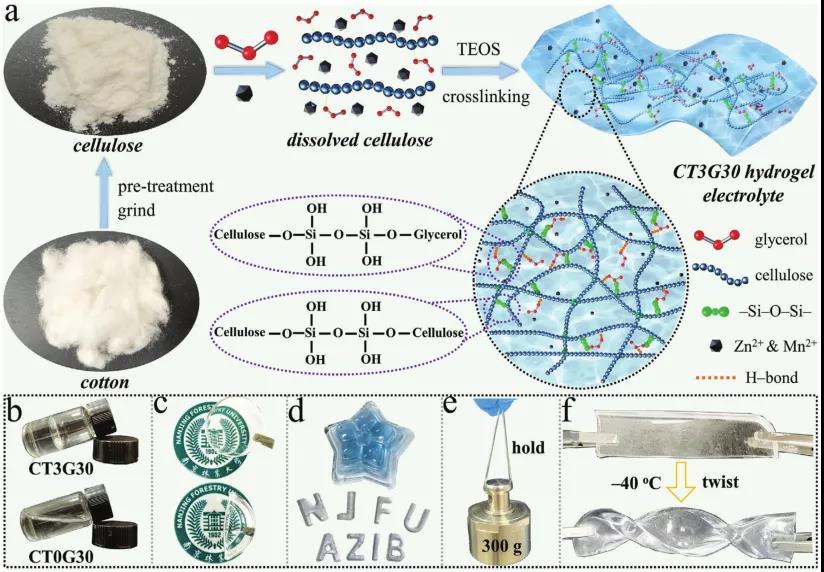
Figure 1. a) Schematic diagram of the synthesis of hydrogel electrolyte.
b) Photos stored in glass bottles.
c-e) Photographs molded into the desired shape under different conditions.
f) Photograph of twisted film at -40°C.
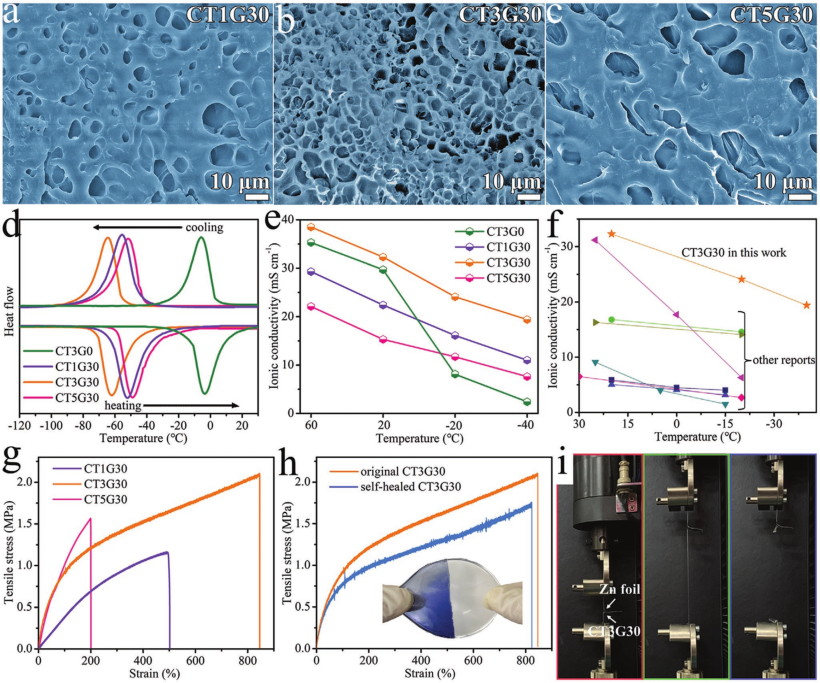
Figure 2. a-c) SEM images of freeze-drying.
d) DSC curve and e) ion conductivity value.
f) Ionic conductivity of antifreeze hydrogel electrolyte
g) Stretch the σ-ε curve.
h) Original and self-healing tensile σ-ε curve.
i) A photo of trying to peel the zinc foil from the film after partially bonding these two components together.

Figure 3.a) Battery cycle performance of CT3G30 at 3Ag-1 and different temperatures.
b) At 20°C and c) at -40°C, before cycling, after 1 cycle and after 2000 cycles, the Nyquist plots of the battery with CT3G30.
d) Under the condition of -40℃ and 0.5Ag-1, the capacity evolution of the battery with CT3G30: under normal conditions and under bending.
e) Demonstration of charging a mobile phone with three batteries connected in series with CT3G30.
f) When the battery is immersed in boiling water, use a CT3G30 battery to power the electronic clock for demonstration.

3. Thesis information
- Previous: Chen Tianfeng ACS Nano
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier
