A review of "JMCA" by Yi Fang, Sun Yat-sen University: Research status and prospects of two-dimensional titanium carbide (Ti3C2Tx MXene)-based electrode materials for supercapacitors
QQ Academic Group: 1092348845
Detailed

Supercapacitors have important applications in many fields due to their fast charge and discharge rate, high power density, long cycle life, and wide operating temperature range. As one of the key components of supercapacitors, electrode materials play a vital role in their performance. MXenes, as a new class of two-dimensional materials in recent years, includes transition metal carbides, nitrides or carbonitrides, usually by selective etching of the parent MAX phase (Mn+1AXn, n=1, 2, 3, 4) "A" in the atomic layer, where "A" is an element in the third or fourth main group of the periodic table (such as Al, Si, etc.), "X" mainly represents C or N elements, and "M" Represents transition metal elements (such as Ti, Mo, Nb, V, etc.). Ti3C2Tx, as a typical MXene material first discovered, has proved to be a very good material because of its abundant surface functional groups, high Youngs modulus, large interlayer spacing, metallic conductivity, and large specific surface area. There are promising electrode materials for supercapacitors. In recent years, research on Ti3C2Tx MXene-based electrode materials for supercapacitors has developed rapidly.
Based on this, Professor Yi Fangs group from the School of Materials Science and Engineering, Sun Yat-sen University published a review article titled "Ti3C2Tx MXene for electrode materials of supercapacitors" in Journal of Materials Chemistry A. This review summarizes the research progress of Ti3C2Tx MXene applied to supercapacitor electrode materials, and focuses on its excellent electrochemical performance and related mechanisms. The preparation and synthesis strategies of Ti3C2Tx-based supercapacitor electrode materials, electrode microstructure, electrochemical properties, charge storage mechanism, mechanical properties, advantages and disadvantages are systematically discussed. In addition, the current problems, possible solutions and future development prospects of Ti3C2Tx-based supercapacitor electrode materials are described.
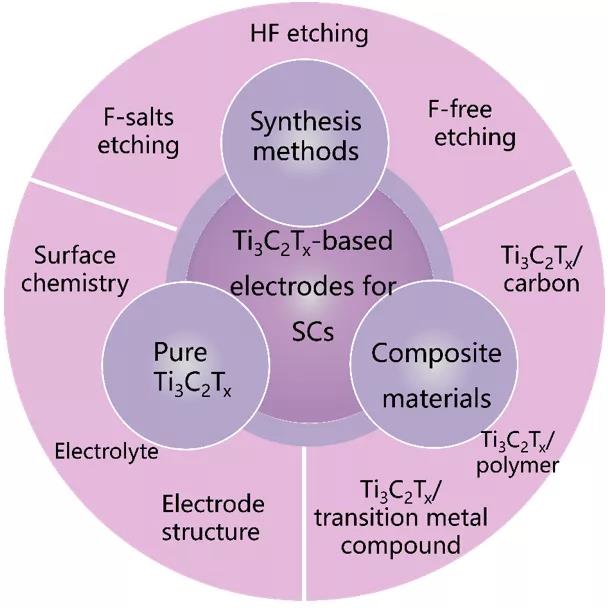
Figure 1. The main content framework of this review article
Summary of article content:
The preparation method of Ti3C2Tx is mainly divided into F-containing etching method and F-free etching method. Different preparation methods have their own advantages and disadvantages. For example, HF can be directly etched to prepare Ti3C2Tx. Although the operation is relatively simple, HF is very dangerous, and the etching products generally have many defects. LiF+HCl is used as an etching agent, the etching conditions are relatively mild, and the prepared Ti3C2Tx thin slices have fewer defects and larger sizes. However, fluorine-containing etchant will affect the performance of Ti3C2Tx specific capacitance. The fluorine-free synthesis strategy can effectively avoid the introduction of -F surface groups, thereby improving the surface electrochemical performance of the Ti3C2Tx sheet. For the practical application of Ti3C2Tx MXene, a suitable etching method is very important.
In order to prepare high-quality Ti3C2Tx-based supercapacitor electrode materials, methods such as cation intercalation, surface functional group modification, and three-dimensional porous or layered electrode structure construction are generally used to obtain high specific capacitance. The Ti3C2Tx electrode can be modified by surface functional groups to obtain higher -O content and lower -F content, which will help store more charges; constructing a three-dimensional porous or layered Ti3C2Tx electrode structure can reduce the agglomeration of two-dimensional nanosheets. Possibility to obtain more effective active sites, thereby improving electrochemical performance; In addition, compared with neutral, alkaline and organic electrolytes, due to the excellent conductivity of H2SO4 and the smallest cations (protons), Ti3C2Tx electrodes are more acidic The electrolyte exhibits a higher capacitance value, and in the organic electrolyte, the Ti3C2Tx electrode can obtain a wider potential window, which is conducive to obtaining a higher energy density.
In practical applications, in order to expand the scope of application and further improve the electrochemical performance, flexibility and even stretchability of Ti3C2Tx-based supercapacitor electrodes, Ti3C2Tx can be composited with carbon materials, polymers and metal compounds and other materials to prepare Composite supercapacitor electrode material. (1) Self-assembly method, layer-by-layer assembly method, surface in-situ growth method and other methods can be used to introduce carbon materials into Ti3C2Tx, which can not only make the electrode have a larger specific surface area and wider interlayer spacing to obtain better Rate performance, and good flexibility can be obtained due to the strong interaction between the surface groups of the two materials. (2) Ti3C2Tx/conductive polymer composite electrode materials can be prepared by polymer monomers on the surface of Ti3C2Tx nanosheets. They exhibit higher specific capacitance, good mechanical flexibility and longer cycle life. It is beneficial to the higher pseudocapacitance and excellent flexibility of the conductive polymer, as well as the good cycle stability of Ti3C2Tx. (3) The Ti3C2Tx/metal compound composite electrode can be prepared by depositing a transition metal compound on the surface of the Ti3C2Tx sheet. The excellent pseudocapacitance of the transition metal compound helps to increase the specific capacitance of the composite electrode, and the excellent conductivity of Ti3C2Tx helps For these composite electrodes, better rate performance is obtained.
So far, Ti3C2Tx has shown great application potential in supercapacitor applications, but there are still many challenges. First, Ti3C2Tx-based electrode materials for supercapacitors are still in their infancy, and there is still a long way to go to achieve large-scale commercial applications. Therefore, the study of low-cost, high-yield Ti3C2Tx electrode material preparation strategies, reasonable electrode structure design methods, etc. have important practical application significance. Second, the oxidation stability of Ti3C2Tx will directly affect the electrochemical performance of electrode materials. Low temperature/inert atmosphere storage and surface modification are effective strategies to solve this problem. It is still very important to study how to better improve the oxidation stability of Ti3C2Tx. important. Third, for Ti3C2Tx three-dimensional porous or layered electrode structures, although they have a large specific surface area and fast ion mobility, the existence of too many void structures will cause them to face the problem of low volume specific capacitance. In the research, the electrode structure should be optimized to minimize the ineffective space of the capacitor and increase the electrode packing density, so as to obtain a higher volume-specific capacitance. Fourth, for the application of Ti3C2Tx-based supercapacitor electrode materials in the field of flexible electronics, better mechanical flexibility is often accompanied by the sacrifice of electrochemical performance. Therefore, more research may be needed to find the relationship between electrochemical performance and mechanical performance. balance. Fifth, standardized performance indicators can bring more efficient academic exchanges. In order to compare the performance of various Ti3C2Tx-based supercapacitor electrode materials more intuitively and scientifically and promote related research progress, it is necessary to establish more scientific and standardized test standards and specification.
Original link:
https://doi.org/10.1039/D1TA00681A
- Previous: ACS Nano: Highly orien
- Next: MXene breakthrough: Na


 mxene academic
mxene academic