The team of Professor Feng Jinkui of Shandong University ACS NANO: Design a three-dimensional sulfur-doped MXene/ZnS heterostructure as a multifunctional protective layer for zinc anode
QQ Academic Group: 1092348845
Detailed

Article information
Design a three-dimensional sulfur-doped MXene/ZnS heterostructure as a multifunctional protective layer for zinc anodeFirst author: An Yongling Corresponding author: Feng Jinkui Unit: Shandong University
Research Background
Because zinc anode has many advantages such as high theoretical reversible capacity, low oxidation-reduction potential, low cost, abundant resources, and environmental friendliness, water-based zinc metal batteries are widely regarded as one of the promising candidate batteries. However, uneven zinc deposition can cause severe dendrite growth and huge volume expansion, resulting in low Coulomb efficiency and short circuits. In order to solve the problem, researchers have proposed many methods to extend the service life of zinc anodes, such as introducing an interfacial electric field, adjusting the coordination environment, and inducing uniform zinc deposition. However, these methods are more complicated and difficult to implement on a large scale. Therefore, there is an urgent need to propose a simple and effective method to inhibit the growth of zinc dendrites.
Article Introduction
Because zinc anode has many advantages such as high theoretical reversible capacity, low oxidation-reduction potential, low cost, abundant resources, and environmental friendliness, water-based zinc metal batteries are widely regarded as one of the promising candidate batteries. However, uneven zinc deposition can cause severe dendrite growth and huge volume expansion, resulting in low Coulomb efficiency and short circuits. In order to solve the problem, researchers have proposed many methods to extend the service life of zinc anodes, such as introducing an interfacial electric field, adjusting the coordination environment, and inducing uniform zinc deposition. However, these methods are more complicated and difficult to implement on a large scale. Therefore, there is an urgent need to propose a simple and effective method to inhibit the growth of zinc dendrites.
Article Introduction
Based on this, the team of Professor Feng Jinkui of Shandong University published a research article titled "Rational Design of Sulfur-Doped ThreeDimensional Ti3C2Tx MXene/ZnS Heterostructure as Multifunctional Protective Layer for Dendrite-Free Zinc-Ion Batteries" in the internationally renowned journal ACS Nano.By designing the heterostructure of electronically conductive three-dimensional sulfur-doped MXene and ion-conductive ZnS, it tries to solve the problems of zinc metal batteries. Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. Based on this, a zinc negative electrode with a long cycle life was obtained.
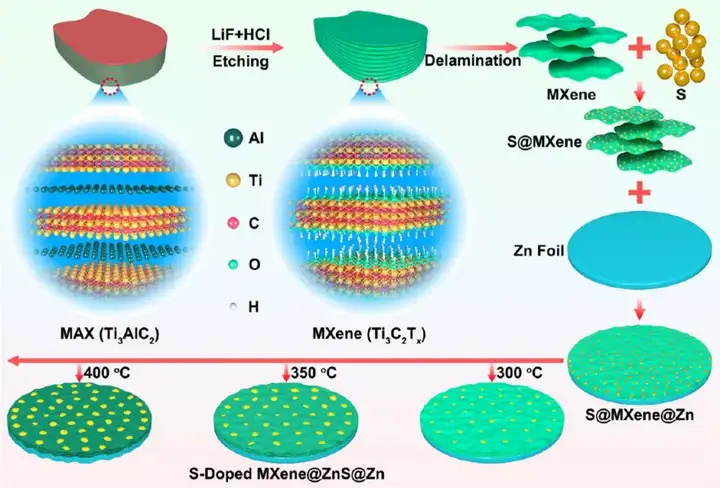
Figure 1 Schematic diagram of synthesis of S/MX@ZnS@Zn at different temperatures.
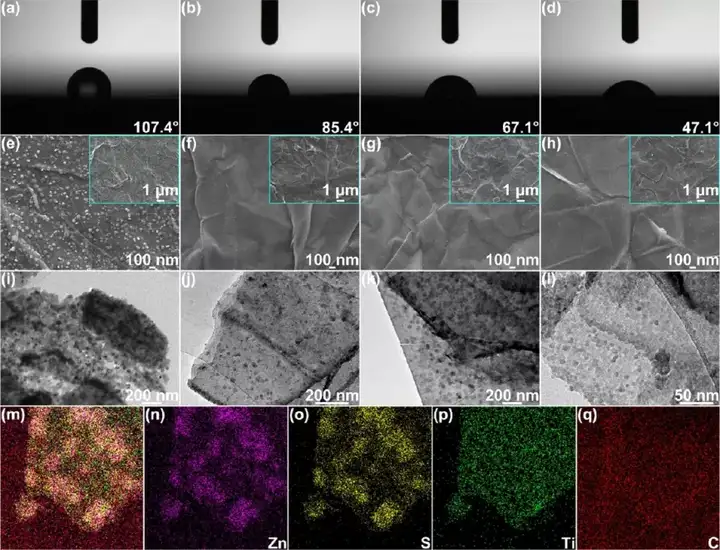
Figure 2 Synthesis and characterization of the product S/MX@ZnS@Zn. (Ad) (a) Commercial zinc foil, (b) S/MX@ZnS@Zn-300, (c) S/MX@ZnS@Zn-350 and (d) S/ in 2M zinc sulfate electrolyte The contact angle of MX@ZnS@Zn-400. (E) SEM images of S@MX@Zn, (f)S/MX@ZnS@Zn-300, (g)S/MX@ZnS@Zn-350 and (h)S/MX@ZnS@Zn-400 . TEM images of (i) S@MX@Zn, (j)S/MX@ZnS@Zn-300, (k)S/MX@ZnS@Zn-350 and (l)S/MX@ZnS@Zn-400 . (M-q) STEM-EDS element distribution diagram of S/MX@ZnS@Zn-350.
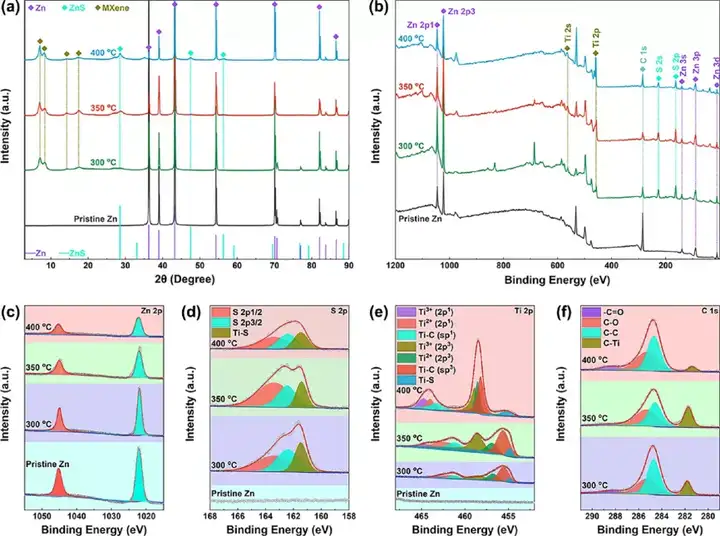
Figure 3 Structural characterization of the product S/MX@ZnS@Zn. (A) XRD diagram, (b) XPS diagram, (c) Zn 2p diagram, (d) S 2p diagram, (e) Ti 2p diagram and (f) C 1s diagram.
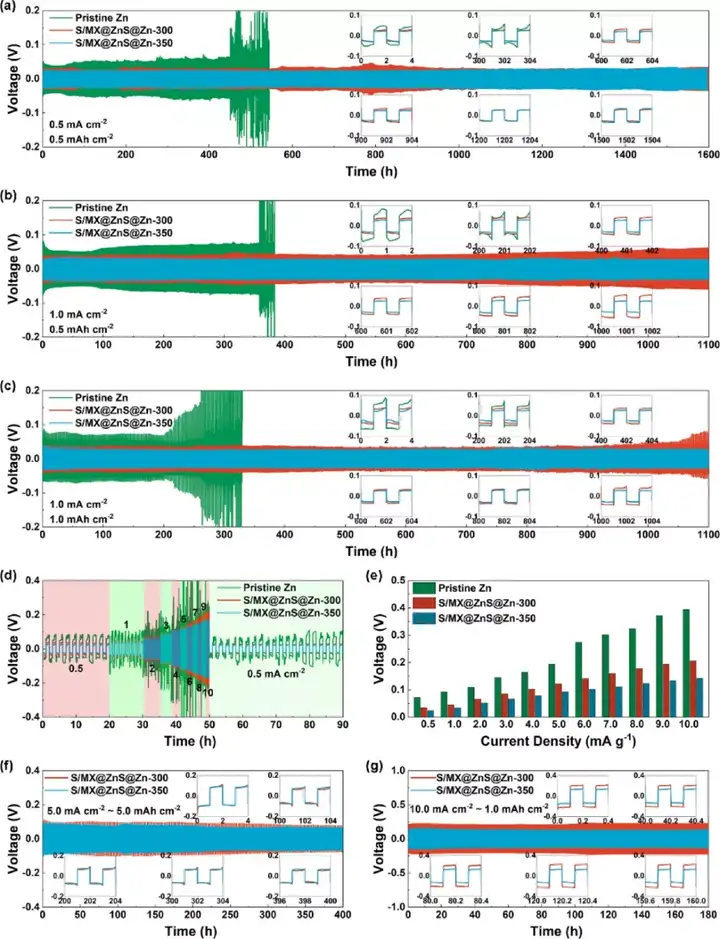
Figure 4 The electrochemical performance of commercial zinc foil and S/MX@ZnS@Zn anode. (A-c) Cycle performance under different current densities. (D) Rate performance and (e) polarization voltage under different current densities. (F-g) Cycle performance under high current density.
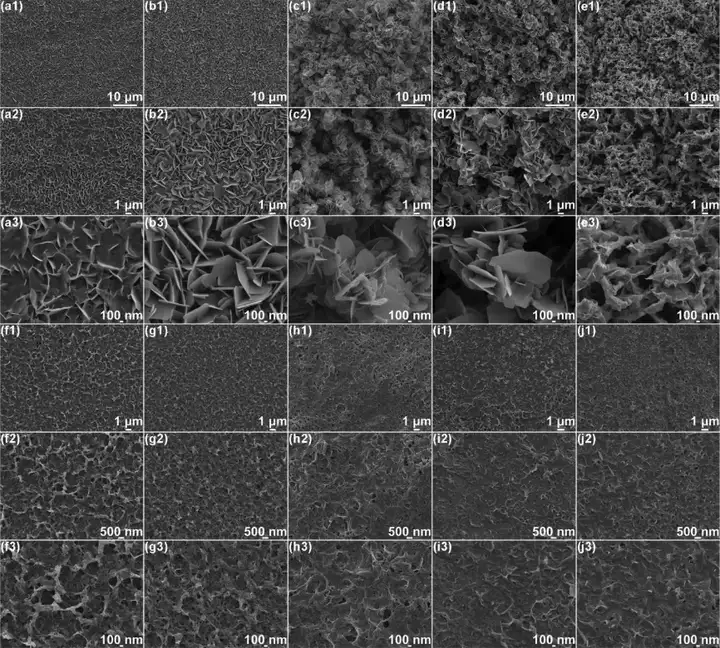
Figure 5 (a-e) SEM images of the structure evolution of commercial zinc foil and (f-j) S/MX@ZnS@Zn anode under different zinc deposits. (A1−a3, f1−f3) 1 h, (b1−b3, g1−g3) 2 h, (c1−c3, h1−h3) 5 h, (d1−d3, i1−i3) 10 h, (e1 −e3, j1−j3) 20 h
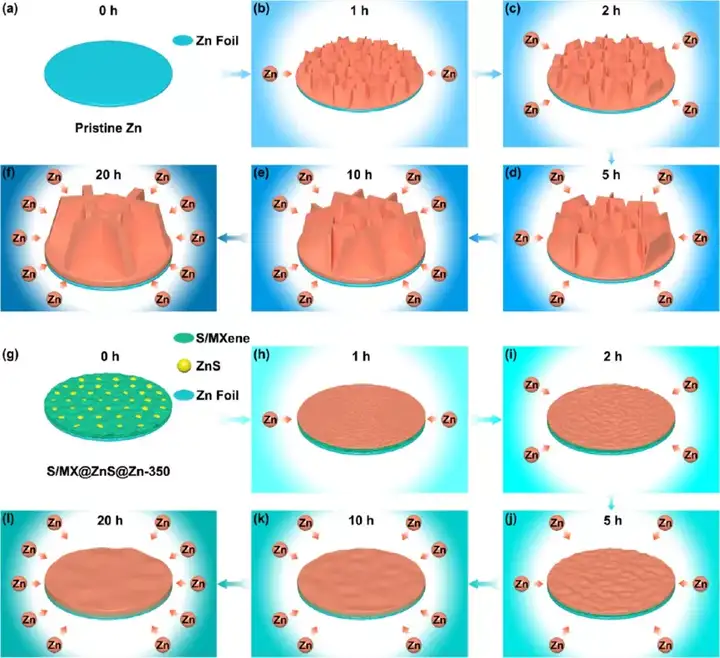
Figure 6 (a-f) Schematic diagram of the structural evolution of commercial zinc foil and (g-l)S/MX@ZnS@Zn anode under different zinc deposits. (A, g) 0 h, (b, h) 1 h, (c, i) 2 h, (d, j) 5 h, (e, k) 10 h, (f, l) 20 h
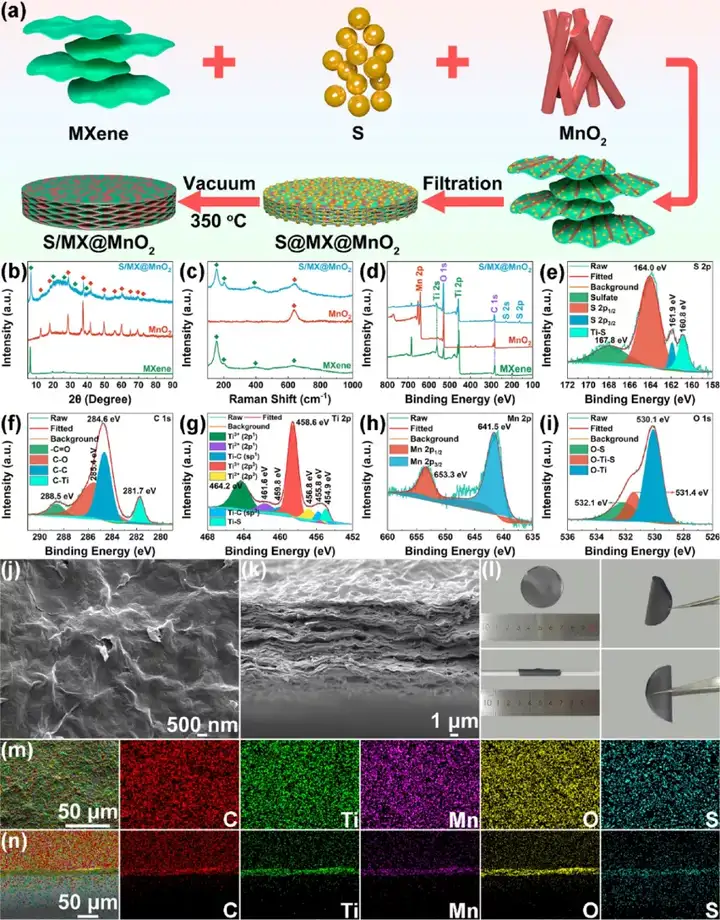
Figure 7 Synthesis and characterization of S/MXene@MnO2 cathode. (A) Synthesis diagram of MXene, MnO2, S/MXene@MnO2. (B) Raman diagram, (c) XPS diagram. S/MXene@MnO2 high magnification (e-i) XPS image, (j-k) SEM image, (l) optical photo image, (m-n) element distribution diagram.
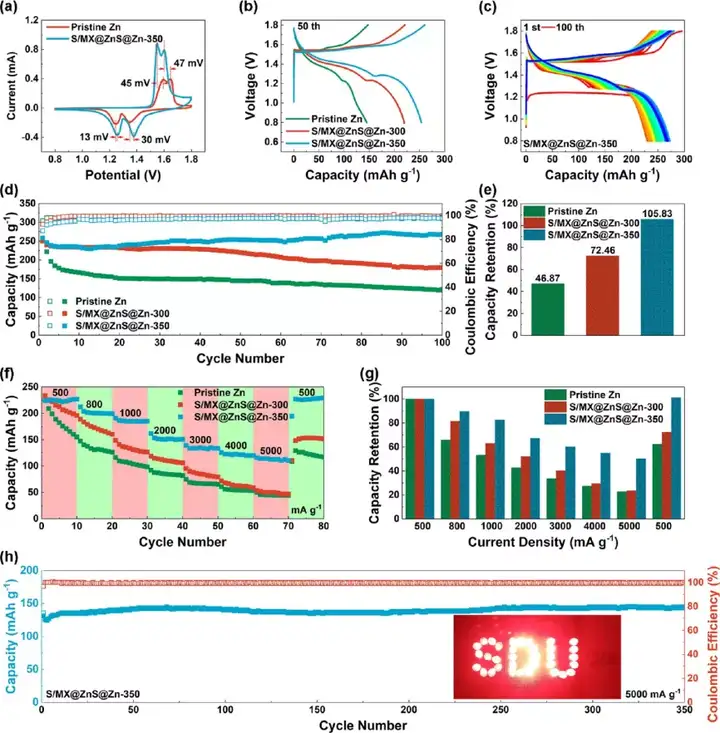
Figure 8 The electrochemical performance of the full battery (the positive electrode is S/MXene@MnO2, and the negative electrode is commercial zinc foil or S/MX@ZnS@Zn). (A) Cyclic voltammetry curve, (b) charge and discharge curve at the 50th week. (C) Charge and discharge curve. (D) Cycle performance and (e) the corresponding capacity retention rate. (F) Rate performance and (g) corresponding capacity retention rate. (H) Long cycle performance.
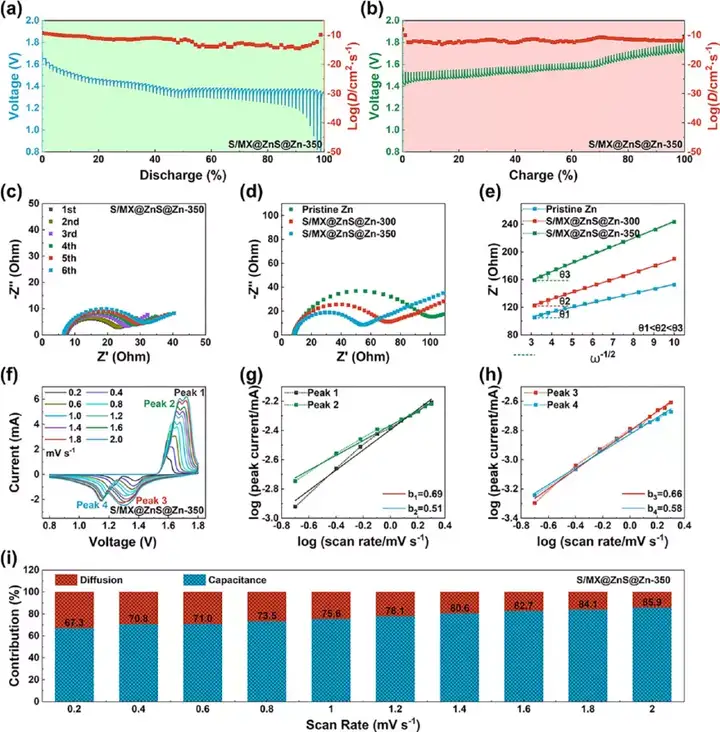
Figure 9 The electrochemical mechanism analysis of the full battery. (A-b) GITT curve and diffusion coefficient. (C) The EIS curve for the first five weeks. (D) EIS curve and (e) the relationship between Z′ and ω−1/2. (F) Cyclic voltammetry curves at different scanning speeds. (G-h) The relationship between peak current and scanning speed. (I) The ratio of pseudocapacitance and diffusion capacitance at different scanning speeds.
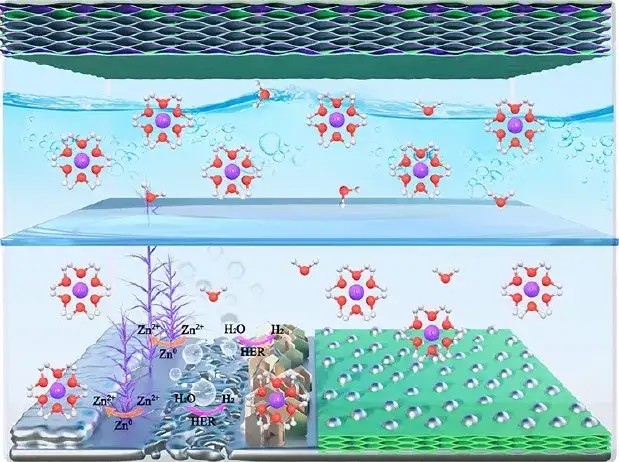
Figure 10 Schematic diagram of water-based rechargeable zinc battery (positive electrode is S/MXene@MnO2, negative electrode is commercial zinc foil or S/MX@ZnS@Zn)
Highlights of this article
1 Construct a multifunctional protective layer for electronic and ion conduction. Through the vacuum distillation method, a three-dimensional sulfur-doped MXene/ZnS heterostructure is obtained in one step. The sulfur doping and porous structure are obtained simultaneously with the synthesis of ZnS. By changing the reaction conditions, the microstructure and electrochemical properties of the product can be adjusted. 2 Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. 3 Build a high-rate water-based zinc-ion battery. With the synthesized self-supporting S/MXene@MnO2 as the positive electrode and S/MX@ZnS@Zn as the negative electrode, a high-rate full battery was obtained.
in conclusion
A multifunctional protective layer with ion and electronic conductivity is constructed and used in water-based zinc metal batteries. (1) Sulfur doping and zinc sulfide production are carried out simultaneously during the preparation process. (2) Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. (3) ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. (4) In water-based zinc ion batteries, the protective layer can exist stably, and the reversibility of the zinc anode can be improved by suppressing side reactions and uniform zinc deposition. Based on the above design, the modified zinc anode can be cycled stably for more than 1600 h. In addition, a self-supporting S/MXene@MnO2 is used as the positive electrode to assemble a high-rate zinc ion battery.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
Highlights of this article
1 Construct a multifunctional protective layer for electronic and ion conduction. Through the vacuum distillation method, a three-dimensional sulfur-doped MXene/ZnS heterostructure is obtained in one step. The sulfur doping and porous structure are obtained simultaneously with the synthesis of ZnS. By changing the reaction conditions, the microstructure and electrochemical properties of the product can be adjusted. 2 Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. 3 Build a high-rate water-based zinc-ion battery. With the synthesized self-supporting S/MXene@MnO2 as the positive electrode and S/MX@ZnS@Zn as the negative electrode, a high-rate full battery was obtained.
in conclusion
A multifunctional protective layer with ion and electronic conductivity is constructed and used in water-based zinc metal batteries. (1) Sulfur doping and zinc sulfide production are carried out simultaneously during the preparation process. (2) Three-dimensional sulfur-doped MXene can homogenize electric field distribution, reduce local current density, and buffer volume changes. (3) ZnS can inhibit side reactions, promote the uniform distribution of zinc ions, and accelerate the transfer of zinc ions. (4) In water-based zinc ion batteries, the protective layer can exist stably, and the reversibility of the zinc anode can be improved by suppressing side reactions and uniform zinc deposition. Based on the above design, the modified zinc anode can be cycled stably for more than 1600 h. In addition, a self-supporting S/MXene@MnO2 is used as the positive electrode to assemble a high-rate zinc ion battery.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
- Previous: Peng Lihua Nano Lett,
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier