Getting Started with Nanomaterial Designs in Implantable Medical Device Batteries
QQ Academic Group: 1092348845
Detailed

core content
1. The system summarizes the working principle, application characteristics and development history of medical implantable disposable batteries.
2. Classified and introduced the various systems of implantable batteries, introduced in detail the main technical barriers of batteries used in the next generation of implantable medical devices, and made the latest research results and schemes on nanotechnology to improve the effectiveness of various types of batteries. analysis.
3. Summarizing the improvement of electrical conductivity through nano-materials, and then the improvement of the overall energy density and charge-discharge cycle efficiency of the battery. The potential applications of nano-materials in medical implantable batteries are discussed.
Implantable medical battery
Implantable medical devices, such as cardiac pacemakers and implanted defibrillators, have extremely high technical requirements for the performance of the disposable batteries they use, such as high energy density, high discharge power, and system stability and safety. From the initial development of nickel-cadmium and zinc-mercury batteries to later lithium-iodine and nuclear (rhenium) batteries, to the latest lithium-carbon fluoride and silver-vanadium oxide batteries, the performance of each generation of implanted batteries is extremely Improvements are helping the rapid development of medical device functions. Under the higher energy requirements and high safety standards of next-generation devices, existing disposable lithium batteries have gradually reached the upper limits of their energy density and power. The research and application of nanomaterial technology in batteries and energy storage devices have received widespread attention in recent years. Therefore, in addition to traditional methods, nanomaterials have great potential in the research and application of next-generation implantable batteries.
Overview
In view of this, Professor Yang Yuan of Columbia University systematically summarized the development history and latest progress of nanomaterials in medical implantable disposable batteries, and how to further improve the electrochemical performance of implantable batteries and improve the battery system‘s performance. Reversibility, as well as strategies to extend life in the human body, are proposed.
Point 1: Lithium-Fluorocarbon Battery
As the most commonly used medical implantable battery, lithium-fluoride battery has the characteristics of high energy density, low self-discharge rate, and relative safety and stability. Under the 3.2V discharge standard, the lithium-fluoride battery can reach a theoretical energy density of 2119Wh / kg and 5830 Wh / L, which is about 4 to 5 times that of a lithium-ion battery. As the main power supply unit for cardiac pacemakers, the lithium-fluoride battery used in the application mainly faces the shortcomings of high internal resistance, low discharge power, and initial voltage drop. Therefore, in actual applications, its average energy is only 440Wh / kg density.
Compared with the carbon materials used for fluorination in traditional fluorinated carbon, the nanoform carbon source significantly improves the ion conductivity. When multi-walled carbon nanotubes were used to fluorinate carbon fluoride, the 12-hour fluorinated sample reached and exceeded 95% of the theoretical energy density. At the same time, the discharge current and charge-discharge cycle efficiency have also been significantly improved. Similarly, when graphene is used for fluorination to produce fluorinated carbon, under the same test standard, a fluorinated carbon sample using graphene as a carbon source has an energy density increase of 40% compared to conventional fluorinated carbon. Carbon nanofibers and disc-shaped carbon nanocarbon sources have significantly improved the energy density and discharge power of batteries.
By reducing the size of the material particles and then increasing the surface area and pore volume, the electrochemical activity of the fluorocarbon positive electrode can also be improved. This method improves the battery performance especially under high discharge power. In the experimental environment, the carbon fluoride sample after ball milling at 300 rpm has a higher discharge density than conventional carbon fluoride electrodes under the conditions of high discharge currents of 4C and 6C.
The high electrical and thermal conductivity of carbon-based nanostructures, such as carbon nanotubes, is particularly suitable for implantable batteries, so they can also be used as conductive additives, current collectors, and constructing positive electrode structures. After mixing carbon nanotubes (Figure 1d) with fluorinated carbon anode materials, the internal resistance of the anode decreased significantly from> 300ohm to less than 20ohm. When used to build a positive electrode current collector, traditional aluminum current collector samples have basically failed in a high-current pulse test environment (Figure 1e), and fluorocarbon electrodes using carbon nanostructure current collectors still have good energy density. Therefore, when a carbon nanostructure is used to rebuild a sandwich type positive electrode, two layers of carbon nanotubes are wrapped with a fluorocarbon positive electrode on the outside, which further overcomes the high internal resistance of fluorocarbon as a whole. The experimental results (Figure 1f) show a significant improvement in the voltage delay effect of the lithium-fluorocarbon battery.
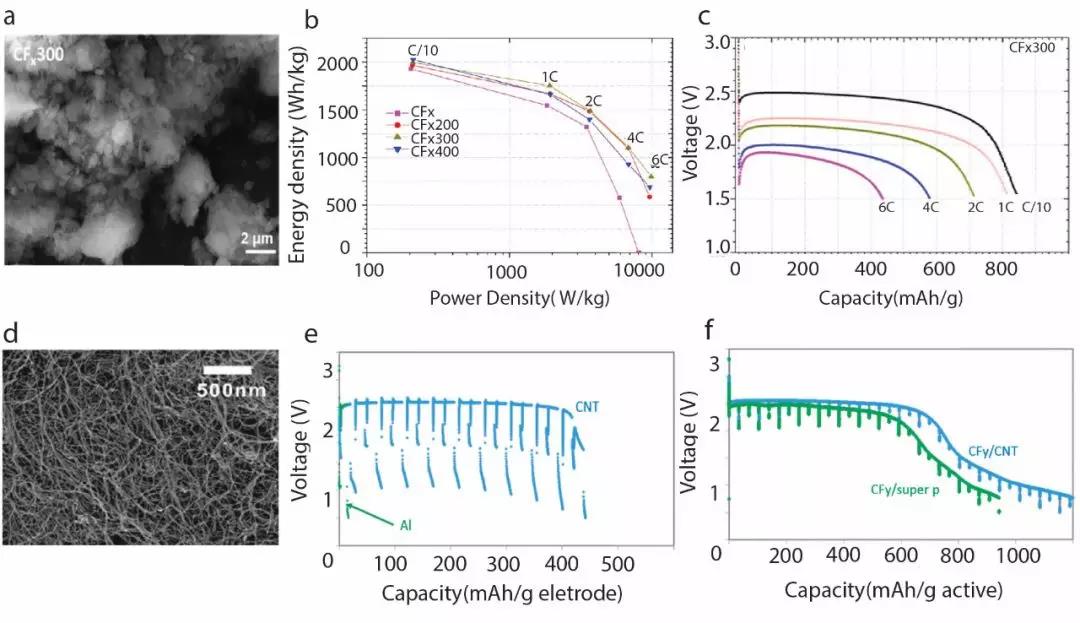 Figure 1. (a) Scanning electron microscope image of a fluorocarbon sample after ball milling at 300 rpm. (B) Energy density of lithium-carbon fluoride samples with different discharge currents at different ball milling speeds. (C) 300 rpm samples at different discharge currents. (D) Scanning electron microscope images of carbon nanotube samples. (E) Comparison of test results between carbon nanotube current collectors and traditional aluminum current collectors. (F) Discharge capacity results of carbon nanotube current collector samples.
Figure 1. (a) Scanning electron microscope image of a fluorocarbon sample after ball milling at 300 rpm. (B) Energy density of lithium-carbon fluoride samples with different discharge currents at different ball milling speeds. (C) 300 rpm samples at different discharge currents. (D) Scanning electron microscope images of carbon nanotube samples. (E) Comparison of test results between carbon nanotube current collectors and traditional aluminum current collectors. (F) Discharge capacity results of carbon nanotube current collector samples.Point 2: Lithium-Silver Vanadium Oxide Battery
Due to the excellent conductivity of silver, lithium-silver vanadium oxide (Li-SVO) batteries can provide up to 10W (2-3A) of pulsed current output and matched energy density. They are often used in implantable cardiac defibrillators. And can provide 5 to 9 years of life. However, compared to lithium-carbon fluoride batteries, the energy density of lithium-silver-vanadium oxide batteries is relatively low, which greatly limits its application in other implanted devices. Therefore, the main application schemes of nanomaterial technology are focused on improving the overall energy density of lithium-silver-vanadium oxide batteries, as well as sustainable charge and discharge performance, and are used for future in vivo charging technology management.
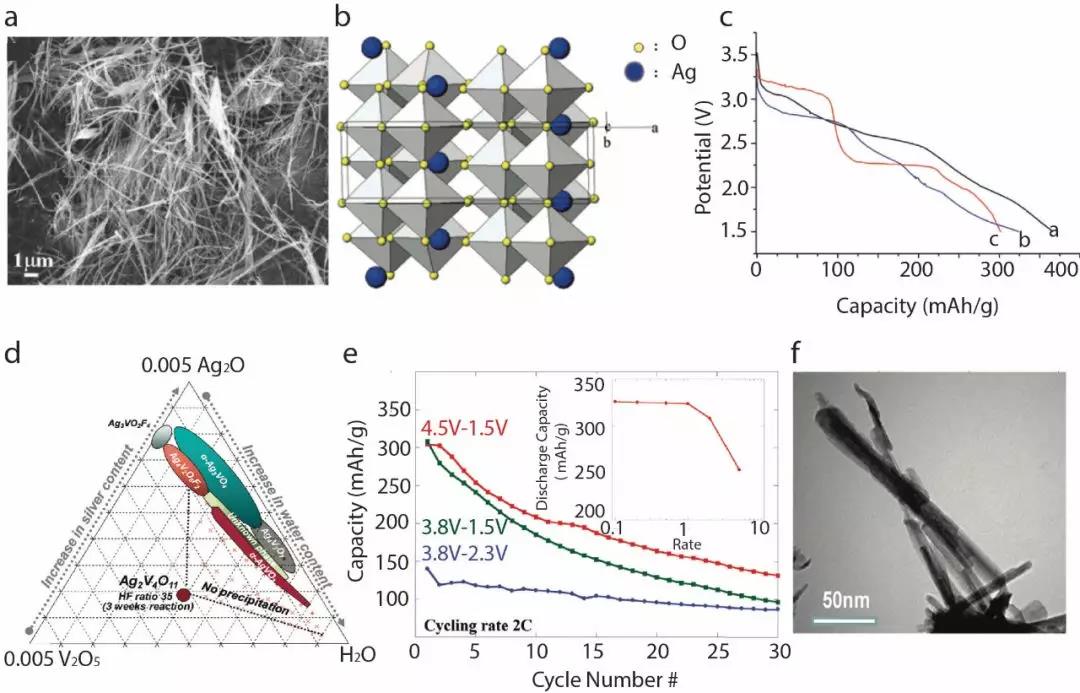
Silver-vanadium oxide nanowires with a diameter of 30-50nm are synthesized by hydrothermal reaction (Figure 2a), which can increase the energy density and open circuit voltage of the battery in a low-current test environment. To further reduce the temperature required for the reaction, a ternary synthesis method (Figure 2d) can be used to synthesize about 10-15 × 50-200 of vanadium pentoxide hydrate, hydrofluoric acid and silver oxide at a known ratio at room temperature. Needle-shaped silver-vanadium oxide nanocrystals in nm size (Figure 2f). In traditional lithium-silver-vanadium oxide batteries, the redox cycle activity of silver ions is poor due to the large size difference between lithium ions and silver ions. Therefore, the traditional lithium-silver-vanadium oxide battery quickly loses 80% of its original capacity in 20 charge and discharge cycles. The sample with needle-like nano SVO crystals as the positive electrode can reduce the SVO size to the nanometer level. This sample not only basically reaches the theoretical discharge capacity of the bulk material under low current test conditions, but the battery capacity performance is significantly improved after 20 charge and discharge cycles (Figure 2e).

Figure 3. (a) Schematic diagram of the structure of polyaniline-coated silver-vanadium oxide nanowires. (b) Battery capacity test results for 20 charge and discharge cycles.
In order to further improve the conductivity of the nano SVO positive electrode, the use of PANi (polyaniline) wrapped silver vanadium oxide nanowires can further improve the electrochemical reaction efficiency in the lithium battery system (Figure 3a). As shown in Figure 3b, different PANI contents have a great impact on the improvement of battery capacity. The black curves in the figure represent the traditional lithium-silver-vanadium oxide samples, and the red, green, and blue represent the different levels of PANi. After 20 charge and discharge cycles, it can be seen that all the samples added with polyaniline have improved in capacity. Among them, the sample with 50wt% polyaniline has the best effect, reaching 200% of the capacity of pure lithium-silver vanadium oxide %about.
Point 3: Lithium-Iodine Battery
Lithium-iodine battery, as the main power supply device for early implantable medical devices, has high safety and stability. In practical applications, it also has excellent specific capacity. In addition, one of the biggest advantages of lithium-iodine batteries is their relatively direct electrochemical reaction. The positive electrode is composed of iodine and PVP (polyvinylpyridine), which produces solid lithium iodide immediately upon contact with the lithium negative electrode, and is used as a battery separator and electrolyte. The biggest limitation of the pure solid-structure lithium-iodine battery comes from its relatively low electrode electrochemical activity and electrolyte ionic conductivity, which limits the battery capacity and discharge power. Industrially, by adding a P2VP (poly 2-vinylpyridine) coating on the surface of a lithium negative electrode, the ion conductivity is improved and this situation is improved. In addition, by introducing nanostructures, the electrochemical reaction efficiency of lithium-iodine batteries can be further improved. When the VACNT (aligned carbon nanotube array) shown in FIG. 4a is used as the current collector of the liquid iodine electrode, the carbon nanotubes provide more space for ion exchange during the charge and discharge process. The initial capacity of the sample battery reached the theoretical capacity of the lithium-iodine battery during the test, and it also performed well in the next 200 charge and discharge cycles (Figure 4b). Despite achieving excellent discharge capacity, the use of liquid iodine as the positive electrode material in implanted devices has the risk of leakage, so the application of this nanostructure in solid positive electrodes is more suitable. During preparation, liquid iodine can be infiltrated into the nanoporous carbon fiber and dried to obtain a solid nanostructure. As shown in Figure 4c, although the remaining capacity of the nanostructured sample decreased after 60 charge and discharge cycles compared to the liquid iodine positive electrode sample, its initial capacity reached 150% of the liquid iodine positive electrode sample.
 Figure 4. (a) Electron microscope scan image of an aligned carbon nanotube array. (B) Capacity test results of a nanostructured liquid iodine positive electrode sample. (C) Capacity test results of nanostructured solid iodine cathode samples.
Figure 4. (a) Electron microscope scan image of an aligned carbon nanotube array. (B) Capacity test results of a nanostructured liquid iodine positive electrode sample. (C) Capacity test results of nanostructured solid iodine cathode samples.
Summary and outlook
As the functions of implantable medical devices become more diverse and diagnostic technology continues to improve, battery power consumption has also increased significantly. By mixing the latest technology of carbon fluoride and silver vanadium oxide to become the positive electrode of the battery, the capacity and discharge power of the implantable battery can achieve the best balance. And this application has been applied in the field of cardiac pacemakers for more than 15 years. Under the constraints of the energy density and capacity of its energy storage components, it is difficult for the next generation of medical equipment to continue to expand more while maintaining a 7-12 year life Features.
The common problem of further improving the electrochemical performance of the above three types of batteries focuses on the electrical reaction efficiency and ionic conductivity, and nanomaterials can reduce the internal resistance of the electrode by effectively increasing the surface area and pore volume without changing its chemical properties. Increase ion exchange efficiency. Therefore, the application of nanomaterial technology has great potential for further improvement of implantable batteries. The above methods of using carbon nanomaterials, nanostructured positive electrodes, and graphene to increase battery capacity and discharge effects have confirmed the possibility of nanomaterials to further improve battery efficiency. At the same time, solid electrolytes and other methods can further enhance the safety and stability of implantable batteries. Combining nanomaterial technology, solid electrolytes, and possible in-body charging methods in the future, the overall safety, stability, and service life of next-generation medical implantable batteries are expected to be greatly improved through nanotechnology.
references
ZhangT, Li Z, Hou W, et al. Nanomaterials for implantable batteries to power cardiacdevices. Materials Today Nano, 2019: 100070
DOI: 10.1016 / j.mtnano.2019.100070
https://sciencedirect.xilesou.top/science/article/pii/S2588842019301385
Source-WeChat public: Nanoman
- Previous: Two-dimensional materi
- Next: 1


 About us
About us