Self-discharge behavior of MXene-based supercapacitors
QQ Academic Group: 1092348845
Detailed
¡¾Research Background¡¿
As an important electrochemical energy storage / conversion device, when high power output or fast energy harvesting is required, supercapacitors are expected to become a substitute for rechargeable batteries. It is well known that the performance of supercapacitors is highly dependent on the inherent characteristics of key electrode materials. MXene has proven to be a promising electrode material for supercapacitors. It shows extremely abundant electrochemically active sites derived from the rapid change of transition metals with different valence states and the rapid redox reaction of surface functional groups. In the past 8 years, it has realized high volume capacitance (1500F cm - 3 ), ultra-high rate performance, fast frequency response (0.12ms, 120Hz), as the energy storage mechanism of MXene-based supercapacitors. However, self-discharge behavior and its mechanism are still a problem in MXene-based supercapacitors.
[Achievement Profile]
Recently, Southwest Jiaotong University Haitao Zhang and Weiqing Yang professor research group at the internationally renowned academic journal ACS Nano published an article entitled: Unraveling and Regulating Self-Discharge Behavior of Ti 3 C 2 T the X- MXene-Based Supercapacitors research papers, which The experimental and theoretical calculations show that the self-discharge mechanism of Ti 3 C 2 T x MXene -based supercapacitors originates from the mixed potential driving process. Experimentally, for the slow self-discharge process, an interface chemical engineering strategy to increase the ratio of bonded ions was proposed.
[Picture and text guide]
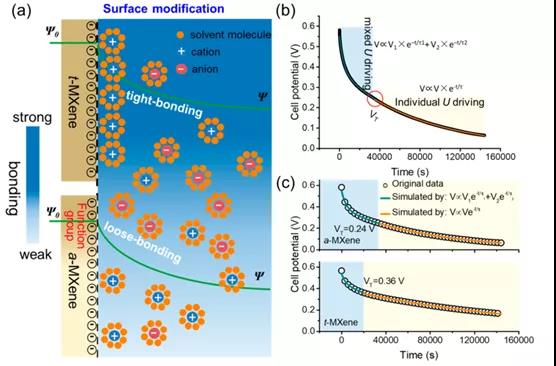
Figure 1. Revealing and adjusting the self-discharge effect of Ti 3 C 2 T x MXene -based supercapacitors
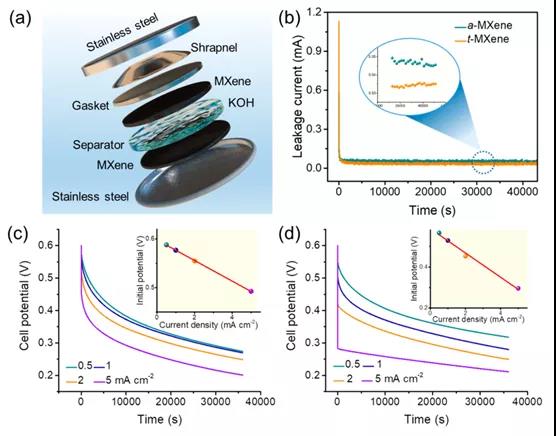
Figure 2. Self-discharge performance of Ti 3 C 2 T x MXene -based supercapacitor
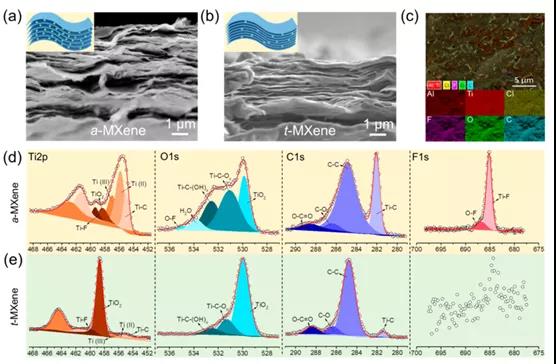
Figure 3. Morphological evolution and composition of a -MXene and t- MXene independent membranes
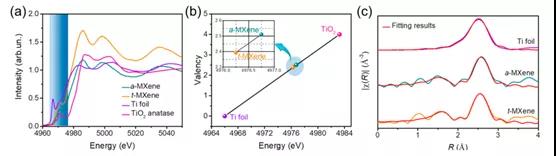
Figure 4. X-ray absorption fine structure of Ti
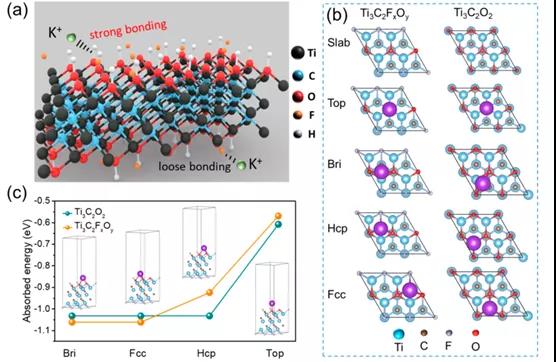
Figure 5. DFT calculation of MXene structure
[Summary of this article]
This paper proposes a mixed potential driving process related to tightly bound and loosely bound ion models to clarify the self-discharge mechanism of Ti 3 C 2 T x MXene-based supercapacitors. To this end, a customized chemical interface strategy is used to enhance tightly bonded ions, thereby effectively regulating the self-discharge process. As a result, the self-discharge rate of Ti 3 C 2 T x t- MXene (˜0.65 atom%) containing a high fluorine element was reduced by about 20% (˜8.09 atom%) compared to a-MXene . XAFS and XPS results show that the improvement of the self-discharge behavior of Ti 3 C 2 T x t -MXene should be attributed to the decrease in average oxidation state. In theory, the DFT results fully reveal that the increase in the adsorption energy between the electrode and the electrolyte ions is the reason for the significant improvement in self-discharge performance. Therefore, this work not only provides an open strategy for customizing the self-discharge process of Ti 3 C 2 T x MXene, but also provides a deeper understanding of the self-discharge mechanism.
Literature link:
https://dx.doi.org/10.1021/acsnano.0c01056
Source:
- Previous£º MXene Flexible Piezore
- Next£º MXene breakthrough: Na


 mxene academic
mxene academic
