【COF 11th】 Controllable synthesis of 3D and 1D metallo-COFs! ! !
QQ Academic Group: 1092348845
Detailed
【Origin】
We know that under the guidance of the concept of dynamic covalent chemistry (DCC) , through the spontaneous correction of structural defects, simple organic building blocks can be connected into highly ordered covalent organic frameworks (COFs). However, the topological structure and possible combinations of building elements currently used to synthesize COFs are extremely limited, which makes it more difficult to create new structures , and thus encourages structural chemists to seek new construction strategies.
Combining the metal coordination concept with DCC is a good way to create COFs structures, and has also successfully obtained some structures (such as woven COF-505-Cu, etc.). However, it is necessary to further understand the interaction between dynamic covalent chemistry and coordination chemistry ! The previously reported woven COFs were synthesized by the covalent condensation of a homoleptic complex and an organic linker. In order to explore the synergy between metal coordination and DCC, this work chose to change the simple organic linker to a polydentate complex , and inhibit or promote ligand exchange by adjusting the coordination form of the metal node (Figure 1), thereby achieving Controllable formation of 3D and 1D metallo-COFs!
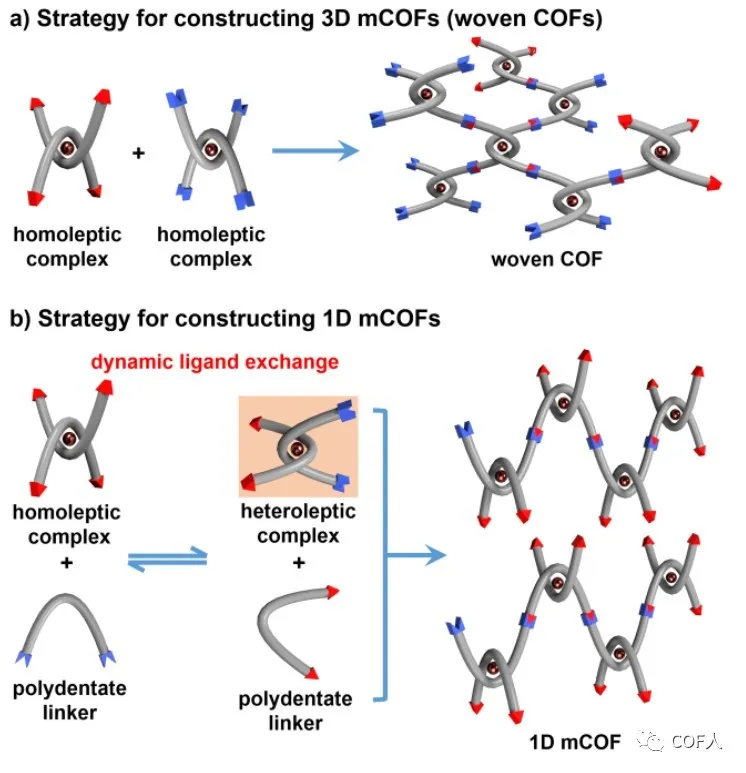
Figure 1 Strategies for constructing 3D-mCOFs (woven COFs) and 1D-mCOFs
[Synthesis chapter 1: 3D-mCOFs]
If the woven COF is constructed from two homogenates, the appropriate structural unit and the stability of the complex under solvothermal conditions are the primary considerations. Therefore, the two complexes shown in Figure 2 were selected for this work . Based on the tetrahedral geometry of the two building blocks, diamond-shaped woven COFs can be expected. Through the imine condensation reaction, the synthesis conditions are: the above two copper complexes in a mixed solution of 1-butanol, 1,2-dichlorobenzene and 6M acetic acid aqueous solution (1/9/1, v / v / v) Heat at 120 ° C for three days. Finally, the crystal structure of W COF-Cu was determined by the combination of three-dimensional electron diffraction (3D ED) and PXRD (Figure 2). The crystal structure was then studied using high-resolution transmission electron microscopy (HRTEM). The ordered lattice fringes from the [022] direction were observed, and the lattice spacing was well matched with the crystal structure.
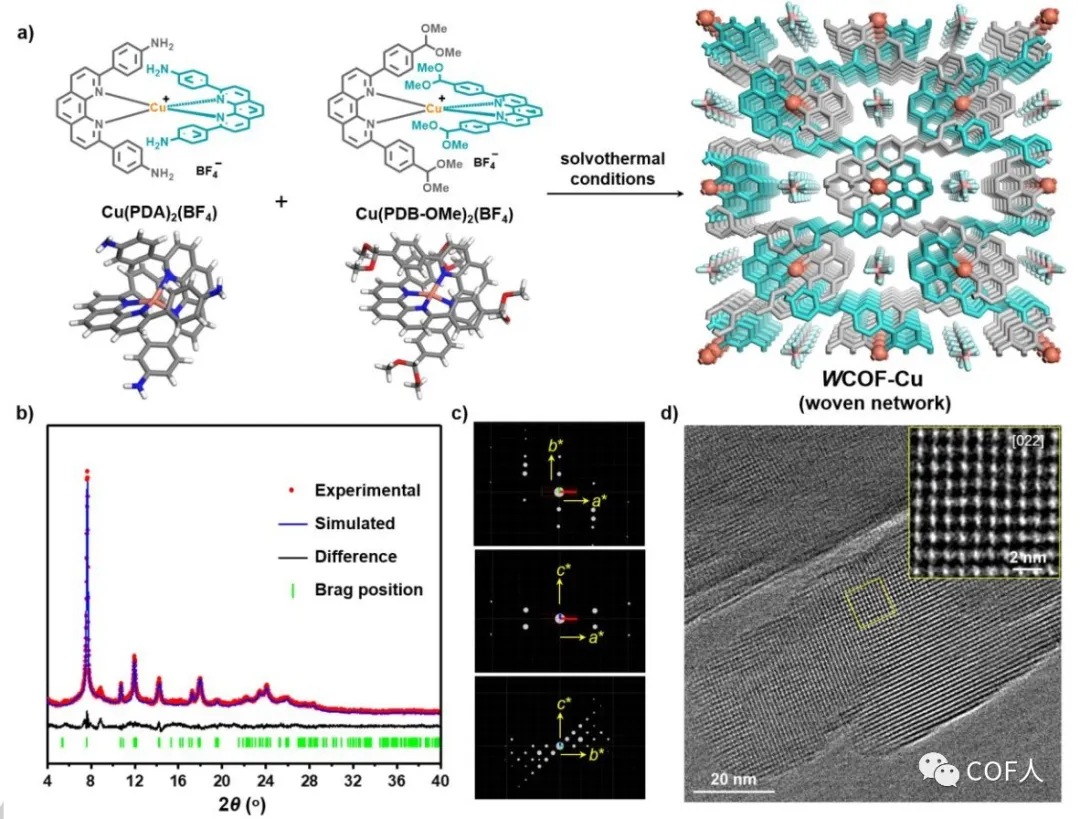
Figure 2 Synthesis and crystal structure of W COF-Cu
[Synthesis chapter 2: 1D-mCOFs]
The above experience of successfully constructing W COF-Cu from homomoleptic homocomplexes (no ligand exchange occurs during crystallization) has prompted the author to explore next whether different structures can be obtained when ligand exchange exists in the system. It is expected that if free ligands containing condensable groups are combined with homogenous complexes, dynamic ligand exchange will occur, which may also result in polycondensation (ML 1 L 2 ) complexes in situ while participating in polycondensation React . Under similar reaction conditions for the synthesis of W COF-Cu, Cu (PDA) 2 (BF 4 ) and 2,9-bis (4- (dimethoxymethyl) phenyl) -1,10-phenanthroline (PDBOMe) as a structural unit, successfully obtained mCOF-Cu (see Figure 3 for details).

Figure 3 Synthesis, crystal structure analysis and characterization of mCOF-Cu
mCOF-Cu may be wider a Cu (a PDA) 2 (the BF . 4 ): the PDB-OMe ratio range (from 1: 1: 2 to 1) is obtained, the presence of which indicates that the local energy minimum under these synthesis window ( small series It can be understood that the structure is stable when the energy is the lowest when driven by heat ). Afterwards, the structure of mCOF-Cu was analyzed using 3D ED and PXRD methods. The results show that the crystal structure of mCOF-Cu is similar to mCOF-Ag. Therefore, based on the single crystal structure of mCOF-Ag (see this public account tweet [the third bomb of COF]), the precise structure of mCOF-Cu is established accordingly . Among them, polymer stacking and crystallization caused by anion bridging and π-π interaction occur simultaneously with polymer chain growth, resulting in the formation of one-dimensional mCOF-Cu.
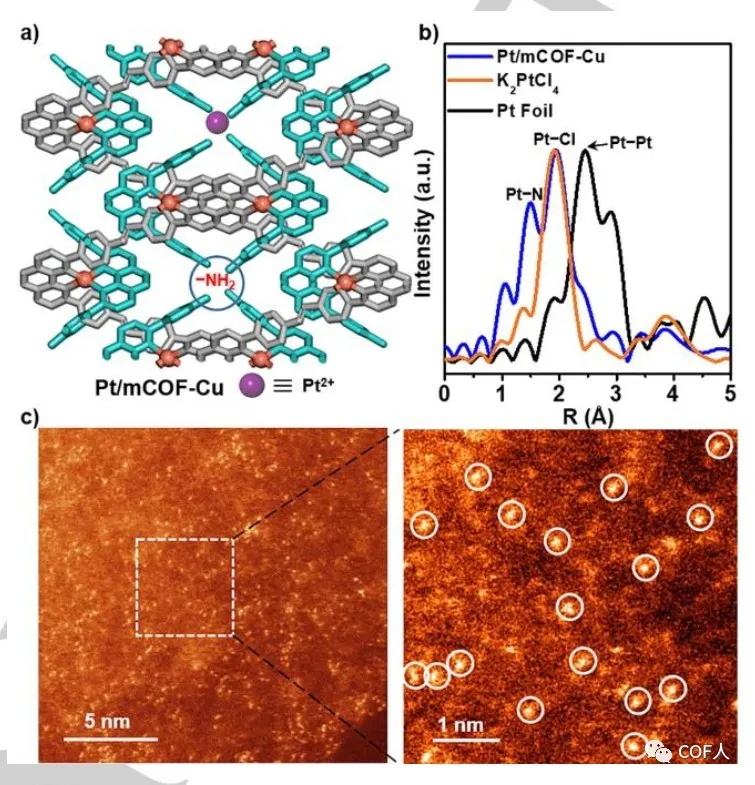
【MCOF-Cu modification】
In one-dimensional mCOF-Cu, the accumulation of zigzag chains and the periodically arranged PDA-suspended amino channels can serve as ideal sites for capturing foreign metals (Figure 4a). The author successfully obtained Pt / mCOF-Cu using potassium chloroplatinate as a Pt source. First, the edge of Pt L3 was analyzed using EXAFS to determine the coordination pattern of Pt (II), and Pt–N and Pt–Cl bonds were detected. Afterwards, aberration-corrected high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) further confirmed the high dispersion of Pt ions . It is proved that this method can be used to manufacture bimetallic centers.
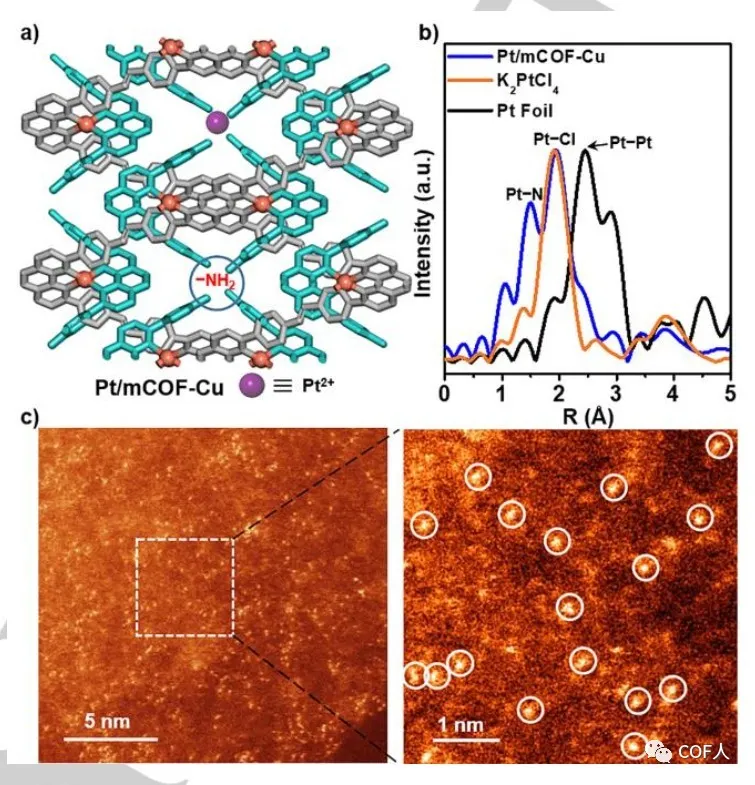
Fig. 4 Structure and characterization of Pt / mCOF-Cu
【to sum up】
This work uses the idea of the interaction of DCC and coordination bonds to guide the synthesis of COF with different structures. 3D woven COF can be generated when using two stable homomoleptic complexes. On the other hand, one-dimensional mCOF can be obtained when the homoleptic complex polymerizes with the free multidentate linker. From a synthetic point of view, this work uses two structural examples to illustrate the synergy of coordination and dynamic covalent chemistry, which can greatly broaden the synthesis and functionalization of COF.
Original link: https://doi.org/10.1002/anie.202002724
Source of information;
- Previous: Yan Dabing team of Hed
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier