Metal-free thiophene-sulfur covalent organic framework: precise and controlled synthesis of oxygen reduction catalytic active center!
QQ Academic Group: 1092348845
Detailed
【Origin】
Defective or heteroatom-doped metal-free carbon materials (MFCMs) have been considered as effective oxygen reduction reaction (ORR) catalysts in the past decade. However, it is difficult to accurately determine and control the active center of ORR in MFCMs using high-temperature pyrolysis or heteroatom doping. In order to verify, as an active center of the catalyst ORR exact construction , the article was first reported two metal-thiophene - sulfur covalent organic frameworks (thiophene-Metal-Free Organic sulfur Covalent frameworks, MFTSCOFs ) as a catalyst ORR.
First, the experimental results show that MFTS-COFs have higher catalytic performance than thiophene-free COFs, indicating that COFs with five-membered ring thiophene-sulfur as the ORR active center have catalytic activity. And, thiophene - the higher the sulfur content of the MFTS-COFs (JUC-528) exhibit better performance ORR addition, uniformly porous structure and a high specific surface area favor the migration of the substance (mass transfer) and a large number of active sites Point exposure also promotes the ORR process.
【synthesis】
The synthesis of MFTS-COFs is based on Schiff base reaction , combining triangular neutral nodes with linear thiophene building blocks.

Figure 1 COF synthesis
【structure】
In this work, FTIR was first used to confirm the successful completion of the designed response, and the precise structure was resolved through PXRD data. The PXRD simulation results and the structure of COFs are shown in Figure 2.
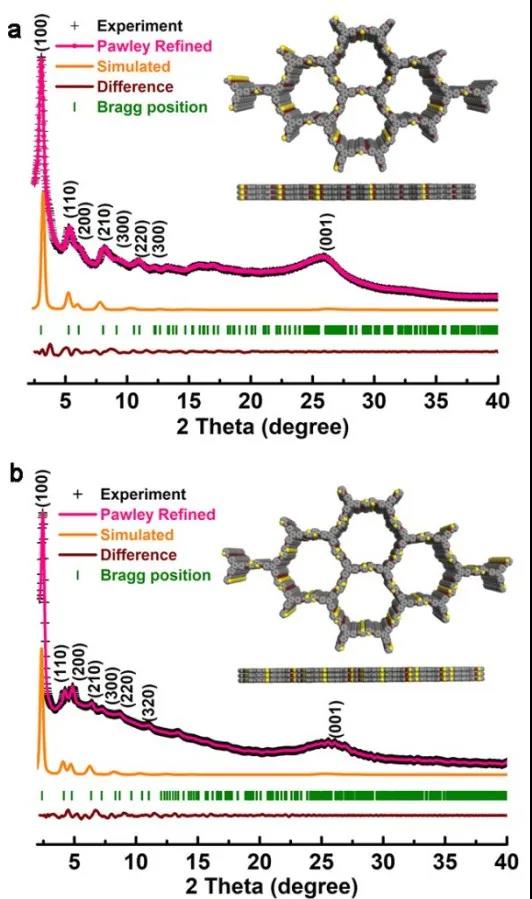
Figure 2 PXRD and structural analysis
【Form】
The morphology of MFTS-COFs was studied with scanning electron microscope (SEM) and transmission electron microscope (TEM), and element-mapping method was used to prove the uniform distribution of sulfur elements in the material.
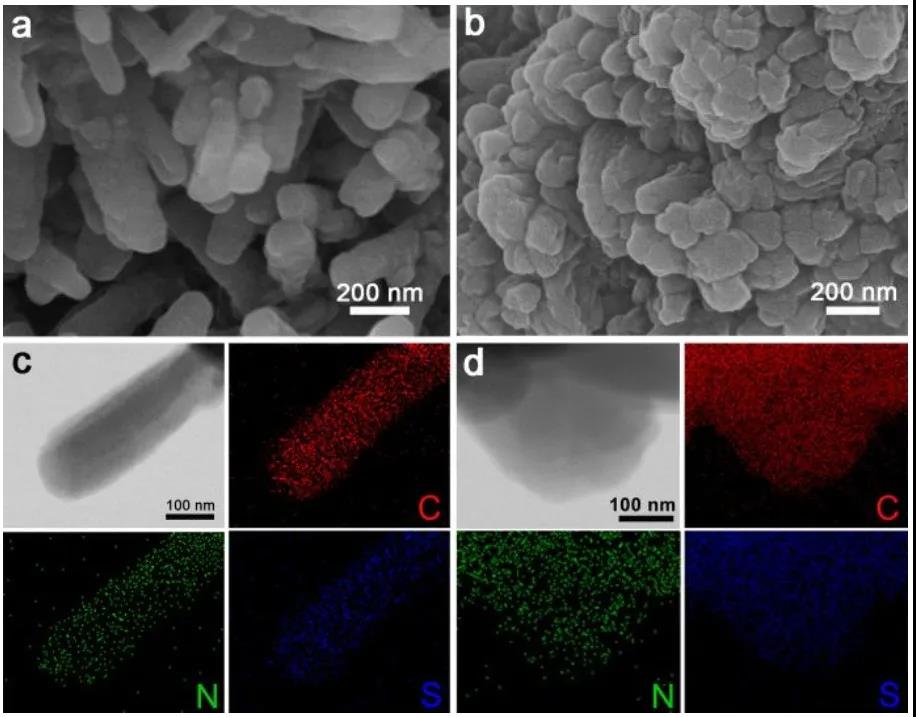
Figure 3 COF morphology characterization
【nature】
On the free thiophene COFs as a control sample together tested for their ORR properties (FIG. 4), demonstrated good or properties containing thiophene, and containing one of the two good ratio (this writing, is not book bags ha hic) . For specific test details and test data analysis, readers are requested to move to the original text.
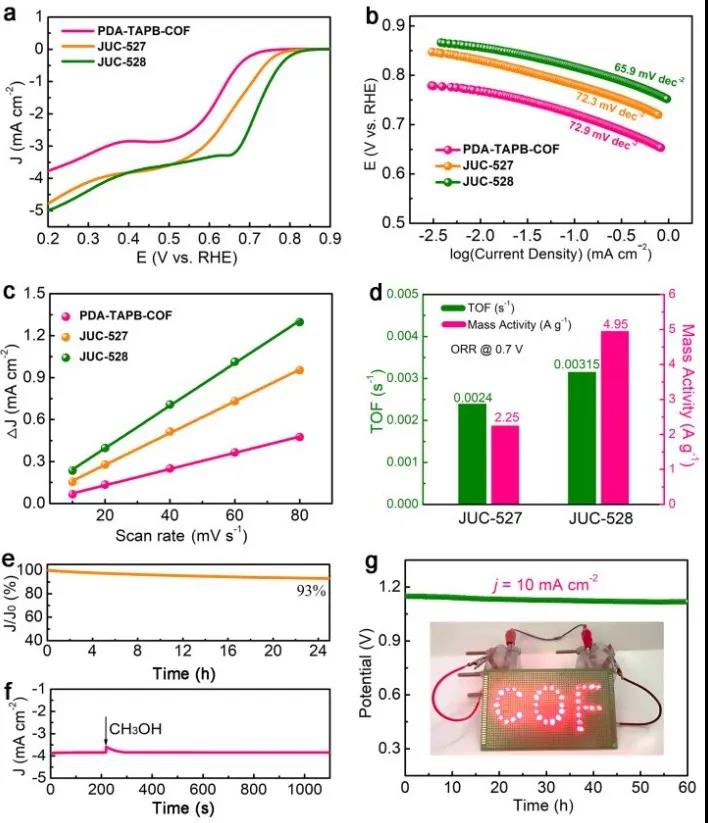
Figure 4 ORR properties of thiophene COFs
【mechanism】
By the density functional theory (DFT) calculations reveal the active site (FIG. 5, the yellow sulfur atom) under basic conditions thiophene rings -S structure is indeed efficient catalytic ORR.
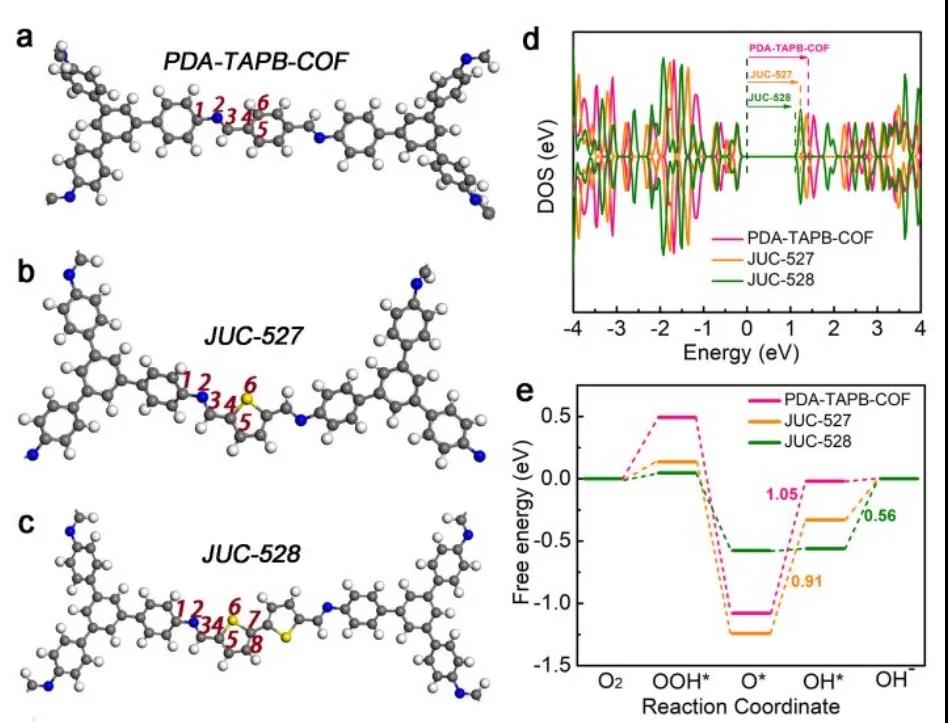
Figure 5 DFT calculation
【to sum up】
In summary, this work designed and synthesized stable metal-free MFTS-COFs using linear thiophene-S as a structural unit (used as an electrochemical catalyst for ORR). It was confirmed that COFs with more thiophene-S structure provided higher ORR activity, and the thiophene-S structure was proved to be the active site by DFT calculation.
Editor ’s comment: In fairness, the content and presentation of this article are relatively simple and easy to understand. The structure of the text is based on [idea source]-[synthesis]-[structure]-[morphology]-[performance]-[mechanism] The order is done in one go! Friendly to readers and worth learning!
Original link: https://doi.org/10.1021/jacs.0c02225
Source of information: COF people
This information originates from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately
- Previous: Adv. Funct. Mater. Of
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier
