Two AEMs a day (1): Professor Wang Guoxiu-MXene separator interface project realizes high-performance lithium selenium and sodium selenium batteries
QQ Academic Group: 1092348845
Detailed
【Introduction】
Good electrolyte-separator interface contact is of great significance for maintaining high capacity and long cycle of the battery. This work improves the electrolyte wettability and ion transmission rate by improving the interface contact between MXene and the membrane, and effectively suppresses the shuttle effect. At the same time, this work for the first time systematically studied the electrochemical behavior of lithium selenium batteries and the protection of lithium metal using in-situ infiltration experiments, which has reference significance for the research of other similar systems.
【Research Background】
With the increasing demand for electric vehicles and grid-scale energy storage, people need higher capacity rechargeable batteries. As a sulfur element, selenium (Se) has been widely studied due to its high theoretical capacity (678 mAh g -1 ) and good electrical conductivity (1 × 10 -3 sm -1 ). However, the severe shuttle effect of polyselenide hindered its development. In recent years, functionalized separators have been prepared to suppress the shuttle effect in selenium or sulfur-based batteries. The MXenes separator has been repeatedly proven to be effective in suppressing the shuttle effect of lithium-sulfur batteries. Nazar et al. Have demonstrated that the surface group of MXene enhances Lewis acid-base chemical adsorption through the formation of thiosulfate. However, this enhanced adsorption undergoes two slow kinetic processes. Although the shuttle effect can be mitigated, the battery exhibits a slow redox reaction, especially at high current densities. In addition, because the surface is rich in hydrophilic groups (-OH and -F), MXene shows a low affinity for organic electrolytes, which in turn leads to poor solid-electrolyte interface contact and slow electrolyte penetration. Therefore, while solving the shuttle effect, it is very important to improve the affinity of the solid-electrolyte interface, which will facilitate ion transport and rapid redox conversion.【Introduction】
Recently, the research group of Professor Wang Guoxiu of Sydney University of Technology in Australia published a research titled " Interface Engineering of MXene Composite Separator for High-Performance Li-Se and Na-Se Batteries " in the internationally renowned journal Advanced Energy Materials (2018 Impact Factor: 24.88) jobs. This work developed a new type of self-assembled MXene composite (CCNT / MXene / PP) separator, which can significantly improve the separator-electrolyte interface characteristics and effectively suppress the shuttle effect. The first author of this article is PhD student Zhang Fan, and Associate Professor Liu Hao is the co-correspondent.
XPS and DFT calculations show that there is a synergistic Lewis acid-base interaction between the MXene composite membrane and the negatively charged polyselenide anion, which greatly improves the adsorption performance of MXene to polyselenide. For the first time, the authors used in-situ permeation experiments in lithium-selenium batteries to study the electrochemical behavior of polyselenide and the barrier effect of MXene composite membranes . At the same time, the increased interlayer spacing due to the intercalation of CTAB molecules in the MXene layer is conducive to rapid ion transport. Introducing carbon nanotubes into MXene nanosheets further improves electrolyte permeability and prevents MXene nanosheets from restacking, and obtains ultra-thin CCNT / MXene / PP membranes with low area mass load ~ 0.09mg cm -2 .
When applied to a lithium selenium battery, the battery showed excellent cycling performance at 1C, 500 cycles, with a capacity decay rate of only 0.05%. The carbon cloth / selenium positive electrode with ahigh selenium area mass load of 5.1 mg cm -2 still maintainsa capacity of485 mAh g -1 after 100 cycles. In addition, MXene composite separators also perform well in sodium-selenium batteries, reaching a high capacity retention rate of 72.6% in 300 cycles.
【Graphic analysis】
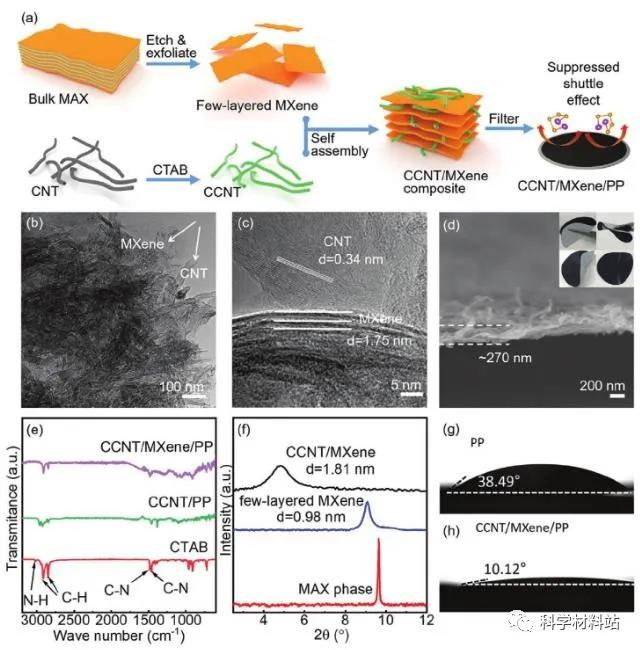
Figure 1. Synthesis characterization diagram
a) Schematic diagram of CCNT / MXene / PP separator synthesis. b) TEM image of CCNT / MXene composite. c) HRTEM image of CCNT / MXene composite. d) SEM cross-sectional view and folding / recovery test of CCNT / MXene / PP separator (illustration) ). e) Infrared spectrum of CTAB, CCNT / PP membrane and CCNT / MXene / PP membrane. f) MAX phase, XRD pattern of few layers of MXene nanosheets and CCNT / MXene composite. g, h) PP and CCNT / MXene / The electrolyte infiltration experiment of PP separator.Figure 1a shows the preparation process of CCNT / MXene / PP separator. On the first CNT by CTAB modified so as positively charged (CCNT), and then mixed with MXene, since the group attached to give CCNT / MXene complex. Finally, CCNT / MXene / PP membrane is obtained by simple suction filtration. The introduction of CNT in the membrane makes the structure more ‘fluffy‘. It is the stacking of CNT and MXene layers, which also provides more active sites for the adsorption of polyselenide. This stacked structure can be well represented in Figures 1b-d, and the stacked membranes show good membrane adsorption and mechanical properties (Figure 1d inset). Secondly, the author verified the composition and proportion of each component in the membrane by means of FT-IR (Figure 1e), Raman (Figure S5), XPS (Figure S4), and the content of CTAB in the diaphragm was only 4.68 wt% . The author verifies here that the presence of CTAB not only modifies the CNT, making it positively charged. At the same time, CTAB also exists between MXene layers, increasing the MXene layer spacing, which will greatly improve the transmission rate of ions between dense MXene layers. Thanks to the addition of CNT and CTAB, the MXene membrane, which is originally rich in hydrophilic groups on the surface, shows a good affinity for organic electrolytes.The authors conducted electrolyte wetting experiments on different separators here, showing that CCNT / MXene / PP composite separators have smaller wetting angles than PP and MXene / PP separators (10.12 ° vs. 38.49 ° and 27.81 °). Thus, it is shown that the CCNT / MXene / PP separator and the electrolyte have better interface contact, which will facilitate the rapid transmission of ions.
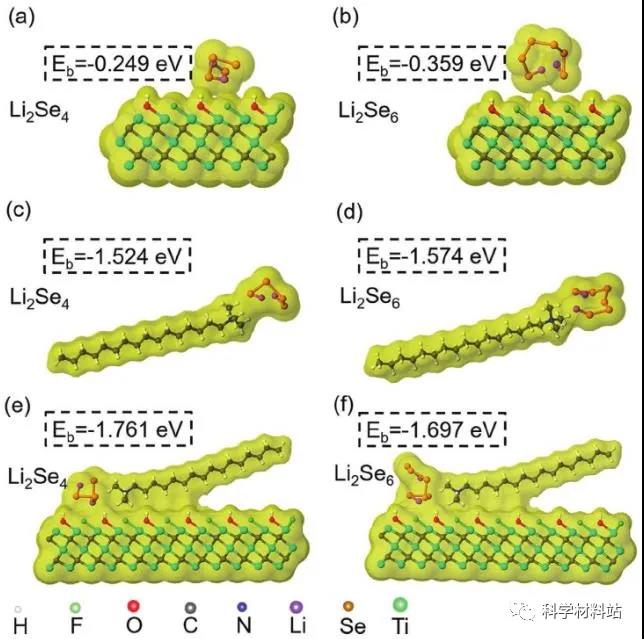
Figure 2. Theoretical calculation
Through first-principles calculations and corresponding binding energies, the optimized Li2Se4 and Li2Se6 models interact with a, b) MXene, c, d) CTAB and e, and f) CTAB–MXene molecular models.
Figures 2a and b show that MXene exhibits relatively weak adsorption strength for Li 2 Se 4 (E b = -0.249ev) and Li 2 Se 6 (E b = -0.359ev ). The authors analyze that this is mainly because the MXene surface groups (-F, -OH, -O) hinder the direct contact between the polyselenide and the active site of Lewis acidic Ti, greatly weakening the binding energy.
In contrast, Figure 2c, d shows that CTAB has a stronger adsorption effect on polyselenide. This strong interaction stems from the close charge attraction between the positively charged CTA + and the negatively charged polyselenide anion. In Figure 2e, f, after CTAB and MXene are combined, CTAB / MXene shows the strongest adsorption to polyselenide compared to the former two. Through analysis, the authors concluded that the anchoring of CTAB molecules on the substrate can change the surface properties of the substrate, for example, increase the wettability and change the local charge density through electrostatic interaction. These modifications may be the reason for the enhanced adsorption of polyselenide on the CTAB-MXene substrate.
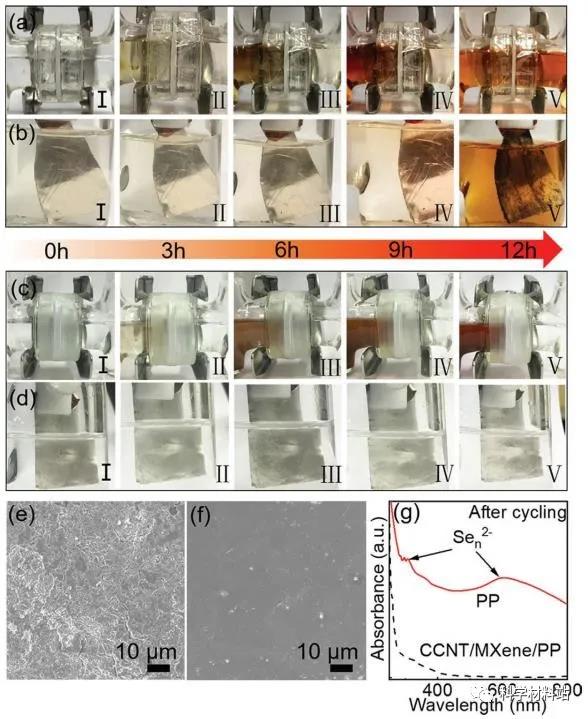
Figure 3. In-situ penetration experiment and ectopic characterization
During the first discharge, in-situ penetration tests were carried out at 0.1C on a, b) PP and c, d) CCNT / MXene / PP separators with H-type batteries for lithium-selenium batteries. SEM images of Li anode of H-type battery after e) PP separator and f) CCNT / MXene / PP separator discharge. g) The ultraviolet-visible spectrum of the electrolyte of the right ventricle of the corresponding H-type battery after discharge.Subsequently, the author further verified the adsorption and blocking effect of CCNT / MXene / PP composite membrane on polyselenide through in-situ permeation experiments . This experiment was carried out for the first time in this system battery and has important guiding significance for the subsequent development of lithium selenium batteries and similar system batteries.
The experiment used H-type batteries ( Figure 3a-d ) to perform in-situ charge-discharge penetration experiments, and intuitively observed the shuttle effect (using PP separator, Figure 3a ) and the suppressed shuttle effect (using CCNT / MXene / PP separator, Figure 3c ) .
Most importantly, the use of the MXene composite separator is beneficial to protect the lithium metal anode (Figure 3b, d, e, and f), which will contribute to the long cycle stability of the battery.
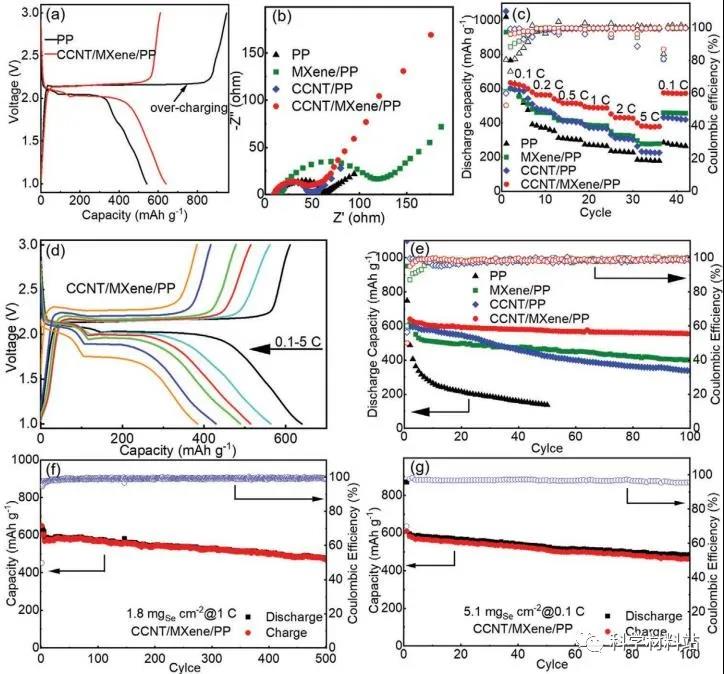
Figure 4. Electrochemical performance of Li-Se battery
Electrochemical performance of Li-Se batteries using different separators. a) The second charge-discharge curve of different separator batteries at 0.1C. b) EIS spectra of different separator batteries before cycling. c) Different diaphragm magnification performance. d) The corresponding rate charge-discharge curve of CCNT / MXene / PP separator battery. e) Cycle performance and 0.1C Coulomb efficiency of different separator batteries. f) Long-term cycling stability of CCNT / MXene / PP membrane at 500 cycles at 1C. g) Cycling stability of CCNT / MXene / PP battery at 0.1C with a high selenium area mass load of 5.1 mg cm -2 for 100 cycles.
Figure 4 shows the electrochemical performance of lithium-selenium batteries using different separators. The results show that the MXene composite separator has better ionic conductivity and lower resistivity. In the end, the author used the MXene composite membrane to achieve better rate performance and long cycle stability. Even under high load (5.1 mg cm -2 ), the battery using the Mxene composite separator still showed stable cycle performance after 100 cycles, and its high capacity was 485 mAh g -1 ( Figure 4g ).
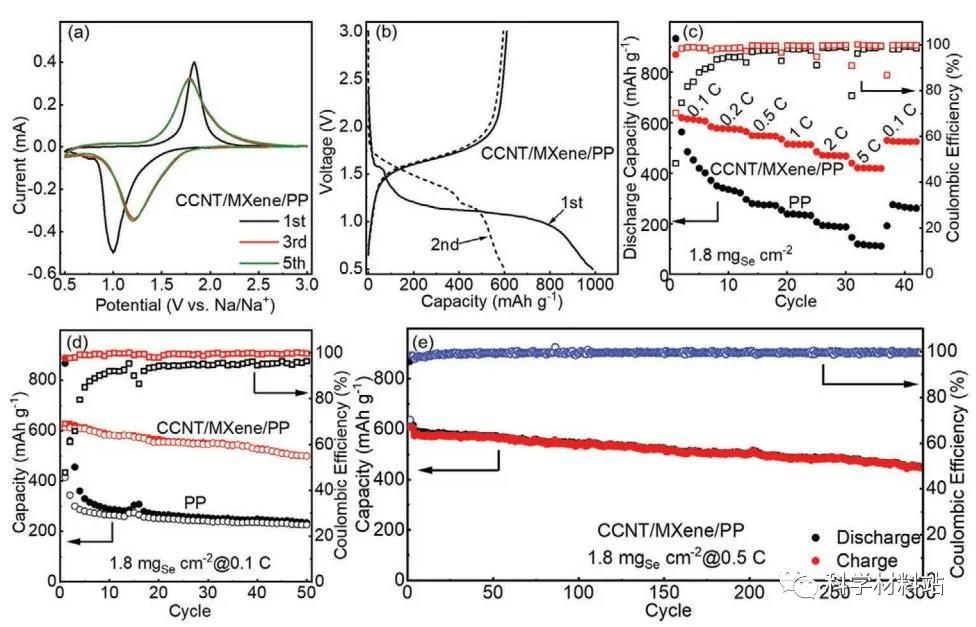
Figure 5. Electrochemical performance of Na-Se battery
a) The CV curve of the CCNT / MXene / PP separator battery in the voltage range of 0.5-3V, b) The first and second discharge-charge curves of the CCNT / MXene / PP separator battery. c) Rate performance of batteries using PP and CCNT / MXene / PP separators. d) Battery cycle performance of PP and CCNT / MXene / PP separators at 0.1C e) Battery cycle performance of CCNT / MXene / PP separators at 0.5C.
【in conclusion】
In summary, the author has designed an ultra-thin CCNT / MXene / PP self-assembled composite membrane. When applied to lithium-selenium and sodium-selenium batteries, the prepared CCNT / MXene / PP separator has an enhanced Lewis acid-base adsorption effect on polyselenides to suppress the shuttle effect. At the same time, the increased distance between the cross-linked CNT network and the MXene layer can improve the wettability of the electrolyte and promote the migration of ions. Through the in-situ penetration test, the electrochemical behavior of selenium during the cycle was studied, which showed that the MXene composite separator played a role in inhibiting the fusiform effect and protecting the lithium anode during the cycle. This work provides a new strategy to establish a better solid-electrolyte interface to suppress the shuttle effect while maintaining fast ion transport.
Source of information:
- Previous: AFM: Overview of MXene
- Next: MXene breakthrough: Na


 mxene academic
mxene academic
