Nature Materials: Lewis acid etching improves the electrochemical performance of MXene non-aqueous electrolyte
QQ Academic Group: 1092348845
Detailed
¡¾Research Background¡¿
Two-dimensional transition metal carbon / carbonitride is a rising star in the 2D material family. MXenes are prepared by selective etching of A-atomic layer elements in the MAX phase, where A represents elements of Group 13-16 (Al, Si, etc.), and M represents pre-transition metal elements (TI, V, Nb, etc.). The general formula is: M n + 1 X n T x , T x represents surface functional groups such as -F, -O and -OH. Thanks to its unique 2D layered structure, hydrophilic surface and metal conductivity (> 6,000 S cm -1 ), MXenes has shown great potential in a wide range of applications, especially electrochemical storage Energy field. With the first report of Ti3C2 MXene in 2011, the main etching environment of MXenes is an aqueous solution containing fluoride ions, such as aqueous hydrofluoric acid, LiF + HCl or ammonium hydrogen fluoride. Up to now, the reactivity of Al, which is mainly derived from Al-containing MAX phase precursors, and fluorine-based aqueous solutions has limited the synthesis of MXenes. Although it has been reported that Si in Ti3SiC2 MAX phase is etched by oxidizer , the etching mechanism is still based on the use of corrosive HF solution. Therefore, the main challenges in the synthesis of MXene are mainly focused on: 1) finding a non-toxic synthetic route; 2) preparing a wider range of MAX phase precursors.
¡¾Achievement Introduction¡¿
Recently, Professor Huang Qing of Ningbo Institute of Materials Science , Chinese Academy of Sciences, Professor Patrice Simon of University of Toulouse, France, and Professor Lin Zifeng of Sichuan University published the title in the top international academic journal Nature Materials : A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non- Research paper on aqueous electrolyte . Different from the traditional method, the paper proposes a method for preparing MXenes by selective etching of A in the MAX phase of non-traditional A element using Lewis acid molten salt, and corresponding verification is carried out. Ti 3 C 2 MXene etched by this method has a reversible capacity of 738 C g -1 (205 mAh g -1 ) and a high coulombic efficiency when applied to lithium storage .
¡¾Graphic introduction¡¿
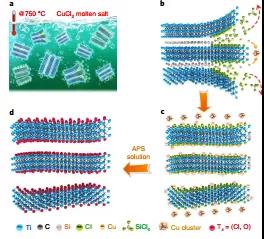
Figure 1. Etching diagram ofMS-Ti 3 C 2 T x MXene.
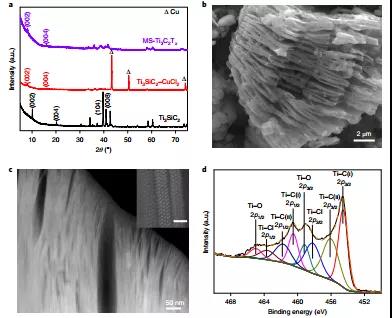
Figure 2. Microstructure and structure characterization of MS-Ti 3 C 2 T x MXene.
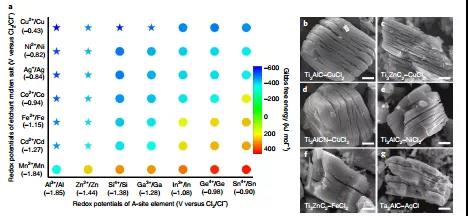
Figure 3. The Lewis acid etching method expands the MAX phase family.
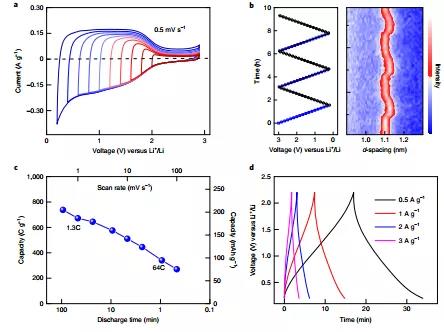
Figure 4. Electrochemical performance test of MS-Ti 3 C 2 T x MXene electrode in 1 M LiPF 6 electrolyte.
¡¾Summary of this article¡¿
Incorporated in a non-aqueous containing of Li + electrolyte of Li + electrochemical energy storage mechanism of insertion and extraction of the "mirror-like" and the high-capacity, high charge-discharge rates and low operating voltage (V FC vs. 0.2-2.2 of Li + / of Li ), So that MXenes obtained by this unconventional etching method has greater potential when used in the negative electrode of electrochemical energy storage devices (batteries and lithium ion capacitors). This general Lewis acid etching method expands the range of MAX phase precursors and can prepare new MXenes materials, providing unprecedented possibilities for regulating the surface chemical properties of MXenes.
Literature link:
https://doi.org/10.1038/s41563-020-0657-0
Source: MXene Frontier
statement:
It is purely academic and non-commercial. If there is any infringement, please contact us immediately. We will delete it as soon as possible to protect the intellectual property of the original author. Thank you teachers and students for your attention and support.
- Previous£º Professor Tao Xutang o
- Next£º MXene breakthrough: Na


 mxene academic
mxene academic
