Nano Energy: Flexible MXenes as a high-capacity cathode material fluoride ion battery
QQ Academic Group: 1092348845
Detailed
Introduction
The new concept of rechargeable fluoride ion batteries (FIB) is safer, cheaper and has higher energy density than traditional batteries. However, due to the higher operating temperature (about 340 K) and the need to select electrolytes and electrodes with excellent electrochemical performance, FIB has not been used in daily life. This paper systematically studied several key parameters of M2CH2 (M = Ti or V) MXene to evaluate its performance as a rechargeable FIB cathode material, including energy stability and thermodynamic stability, electronic properties, strain-driven ions Mobility, average open circuit voltage and theoretical specific capacity.
First confirm the ground state structure of M2CH2, and then study the adsorption behavior of F ions on the substrate. Secondly, with and without strain driving, the F micro diffusion path and migration barrier were determined. Theoretical molecular dynamics (AIMD) calculations prove that F2Ti2CH2 is thermodynamically stable at 500 K. With the combination of these key parameters, we prove that the Ti2CH2 monolayer has the potential to be a flexible and strain-controllable cathode material for rechargeable FIB. This article can provide valuable insights for future experiments to design high-performance, dynamic and stable flexible FIB cathode materials.
Keywords
first-principles calculation; fluoride ion battery; M2CH2 (M = Ti or V) MXenes; positive electrode; strain drive; high electrochemical performance
Background introduction
1. MXene is used in lithium-ion batteries The status quo
is found in graphite with excellent performance After ene, different types of two-dimensional (2D) materials have attracted great interest in the fields of materials science and device processing. By introducing 2D layers of transition metal carbides, nitrides and carbonitrides (called MXene, the molecular formula is Mn + 1Xn (where M is a transition metal, X is C and / or N, n = 1, 2 or 3) They have excellent mechanical flexibility, excellent strength and high electrical conductivity. From an experimental point of view, all MXene is produced by etching the MAX phase in concentrated hydrofluoric acid (HF), which causes the surface of MXene to be , O, OH and a small number of F atoms are functionalized.
These terminating groups give MXene a rich surface chemistry and a highly hydrophilic surface, which has been successfully used in batteries, supercapacitors, electrocatalysts for hydrogen release reactions, ion separation membranes, sensors and electromagnetic interference shielding applications. Regarding electrochemical performance, previous studies have shown that MXene can be stably embedded by various monovalent and multivalent cations, resulting in high volume capacitance.
In addition, it has been proved that the types and functional groups of transition metals show a crucial influence on electrochemical performance. As far as lithium batteries are concerned, the capacitance of the additive-free flexible electrode embedded in Ti3C2Tx is much higher than that of graphite, the main negative electrode material. However, lower capacities were obtained in the Nb2CHx and V2CHx layers respectively. In addition, according to the latest report, the relative abundance of lithium on the earth‘s crust is about 20 ppm, which leads to a relatively high production cost associated with lithium. This poses challenges for scientists around the world to actively develop high energy density, safe, cheap and environmentally friendly energy storage equipment.
2. Advantages of fluoride ion battery
Similar to lithium, fluorine has a high electronegativity and a relatively low weight, so it is very stable and has a wide electrochemical stability window.
More importantly, unlike lithium ion batteries (LIB), fluoride ion batteries (FIB) are safer for users due to less overheating and less environmental impact. Fluoride-containing materials have a fluorite-type structure on a global scale, such as CaF2. Therefore, the new concept of FIB has received widespread attention as a promising alternative to LIB.
In theory, the energy density of FIB can reach more than 5000 W h L-1, depending on the electrode structure.
When fluorine reacts with metal to form metal fluoride, a large change in free energy is observed, resulting in a higher theoretical voltage in the electrochemical cell.
3. Research status of FIB and problems to be solved
Although the concept of FIBs is still in the early stages of development, various metal fluorides such as CuF2, PbF2, BiF3, and KBiF4 have been reported in the literature as cathode materials. Batteries with La or Ce metal as the negative electrode, LaF3 or CeF3 doped as the electrolyte, and PbF2 or BiF3 as the positive electrode show a higher capacity. However, it can only work at a fairly high temperature (about 340 K) and is not practical in daily life. To make matters worse, metal anodes (such as Ce, La, Ca, Li or Mg) are very sensitive to surface oxidation, resulting in rapid degradation of the anode. To date, to achieve the effective function of fluoride electrochemical cells, two key challenges should be solved.
One is about choosing the ideal electrolyte for F-transfer, and achieving significant mass transfer efficiency between the electrode and the electrolyte.
Another problem is how to choose suitable electrodes with excellent electrochemical performance, including ion mobility, open circuit voltage, volume capacitance and cycling performance, and excellent mechanical properties such as elastic stiffness, flexibility and strength.
Recently, a liquid electrolyte for fluoride ion conduction has been reported, which indicates that a fluoride conductor at room temperature can be realized, which will also increase interest in developing new electrode materials. Therefore, searching for ideal electrode materials with suitable electrochemical behavior and mechanical properties plays a vital role in the further development of such secondary batteries.
4. Research status of MXene applied to FIB
MXene has proved to be a promising candidate for LIB electrodes. However, to date, there have been few reports about the chemical embedding or large-scale layering of MXene for FIB.
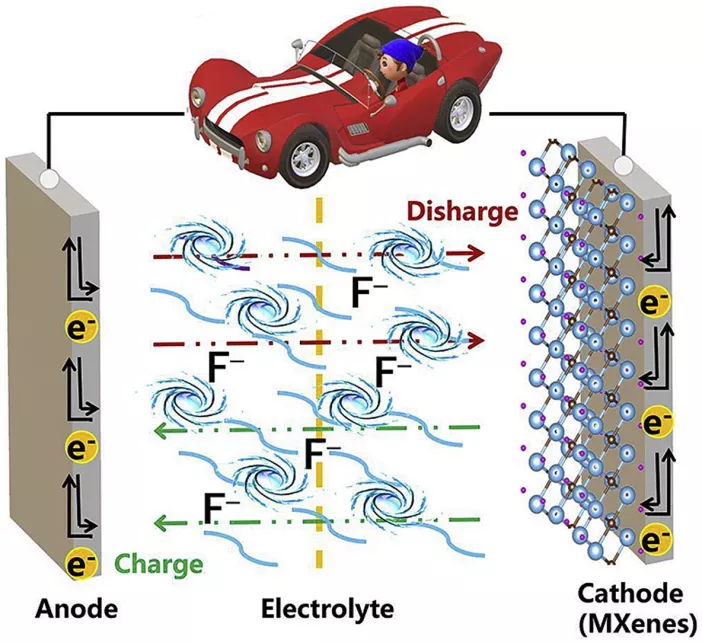
Figure 1. Summary Picture
article describes
based on Nano Energy entitled the above situation, Uppsala University in Sweden Wei Luo and other well-known in the international journal "Fluoride ion batteries: Designing flexible M2CH2 (M = Ti or V) MXenes as high-capacity cathode materials ". Xiao-Yong Yang is the first author of this article.
In this work, the author first studies the electrochemical performance of the representative M2CH2 (M = Ti or V) MXene as the FIBs cathode material. Specifically:
First, the structural stability of M2CH2 with four different configurations is determined by performing first-principle calculations.
Secondly, by analyzing the adsorption energy to determine the most favorable adsorption site.
Then systematically studied the electronic performance and charge compensation mechanism.
In addition, by calculating the migration energy barrier on the possible micro-diffusion path, the F-mobility of the M2CH2 monolayer surface can be predicted. In particular, using the biaxial strain loading scheme (tensile / compression), the F migration energy barrier under various strain states was fully explored. The most important thing is to study the voltage distribution by gradually introducing F on the surface of the M2CH2 monolayer to simulate the F-embedding / de-embedding process and evaluate the corresponding specific energy.
Combining these key electrochemical parameters, some conclusions and suggestions about FIB are drawn.
Article Highlights The
transition metal atom is a key factor in the thermodynamic stability and mechanical flexibility of F2M2CH2 (M = Ti or V) as the electrode material of a fluoride ion battery (FIB).
• In theory, the Ti2CH2 monolayer is a promising cathode material with excellent electrochemical performance of rechargeable FIB.
• The results show that the storage capacity of the Ti2CH2 monolayer in FIB is large (488 (mA h g-1) and the open circuit voltage is high (4.62 V).
• Due to the change in the surface state of the Ti2CH2 monolayer, proper adjustment of the compressive strain can effectively improve F Mobility.
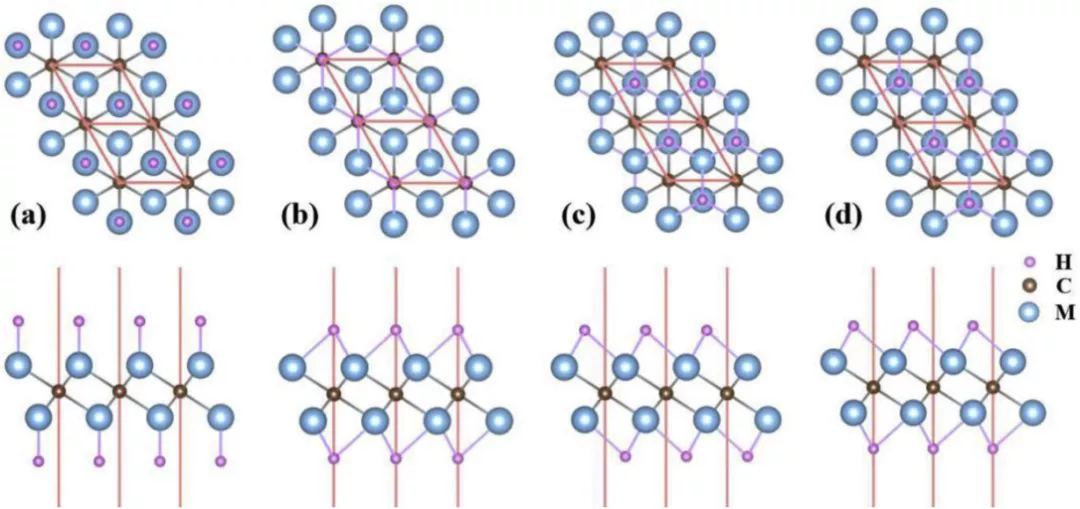
Figure 2. Schematic diagram of M2CH2 structure
Systematic illustration of 1T phase of fully H terminated functionalization M2CH2 (M = Ti or V) MXene system:. Top and side views of (a) MD1, (b) MD2, (c) MD3 and (d) MD4 configuration, respectively
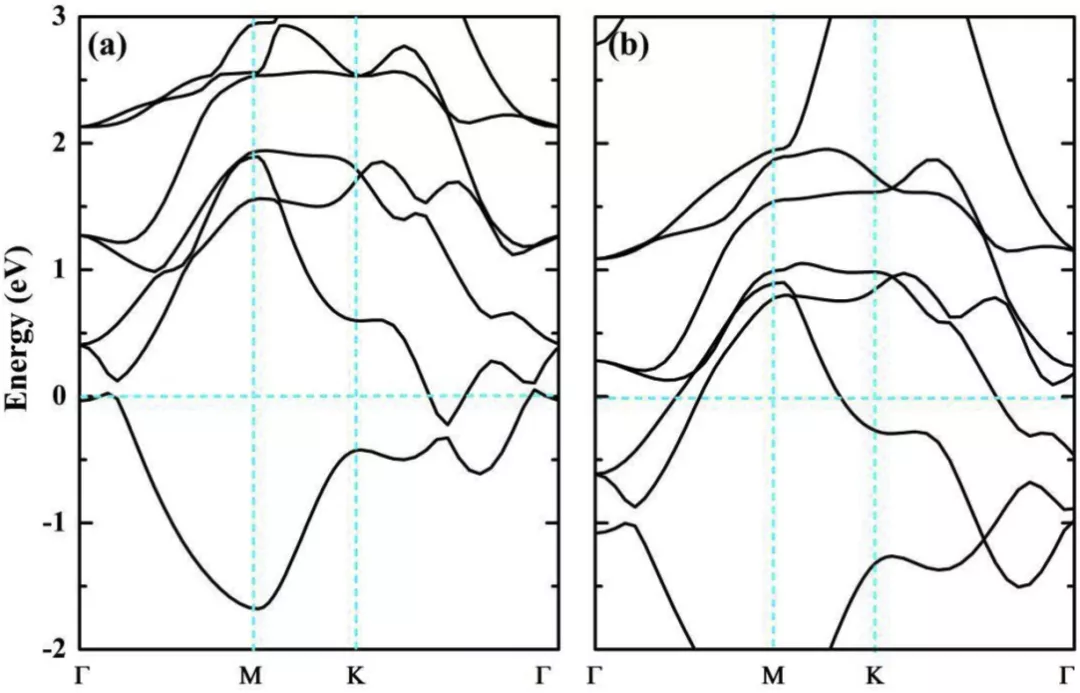
in FIG. 3.
Electronic band structures of (a) Ti2CH2 and (b) V2CH2 in their high-symmetry points. The Fermi level is set to zero.
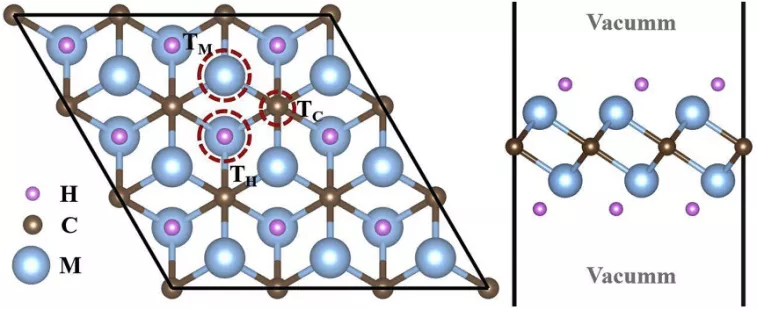
Figure 4. Schematic diagram
of F-adsorption sites Configures of single F- adsorbed on M2CH2 (M = Ti or V) monolayer.
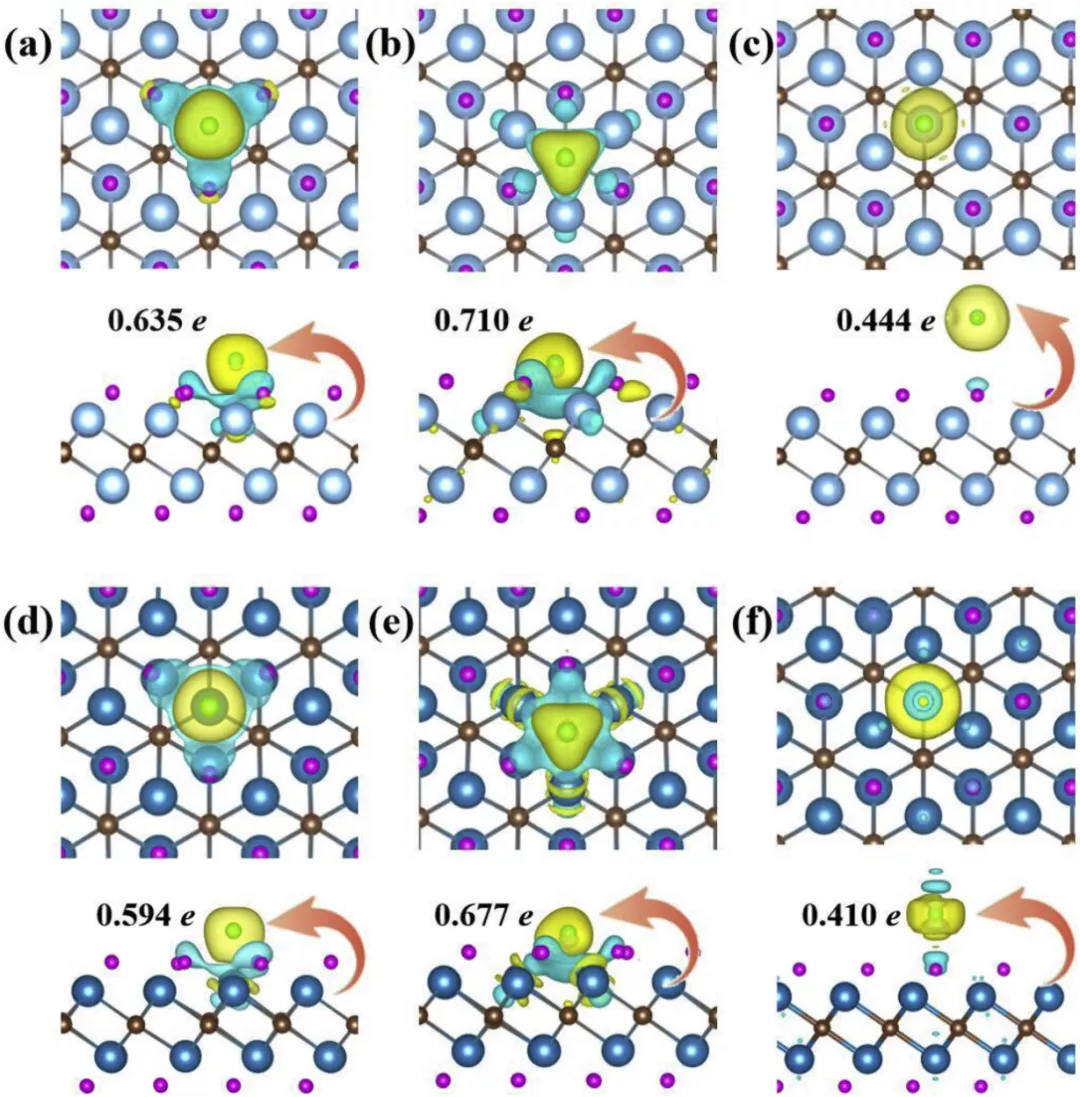
Figure 5. Schematic diagram of F- adsorbed charge density
Charge density differences (CDD) of single F- adsorbed on TM, TC and TH of Ti2CH2 monolayer (upside) and V2CH2 (downside), respectively. Isosurface level set to 0.003 e Å−3, electron-rich regions, red, and electron -deficient regions, blue. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
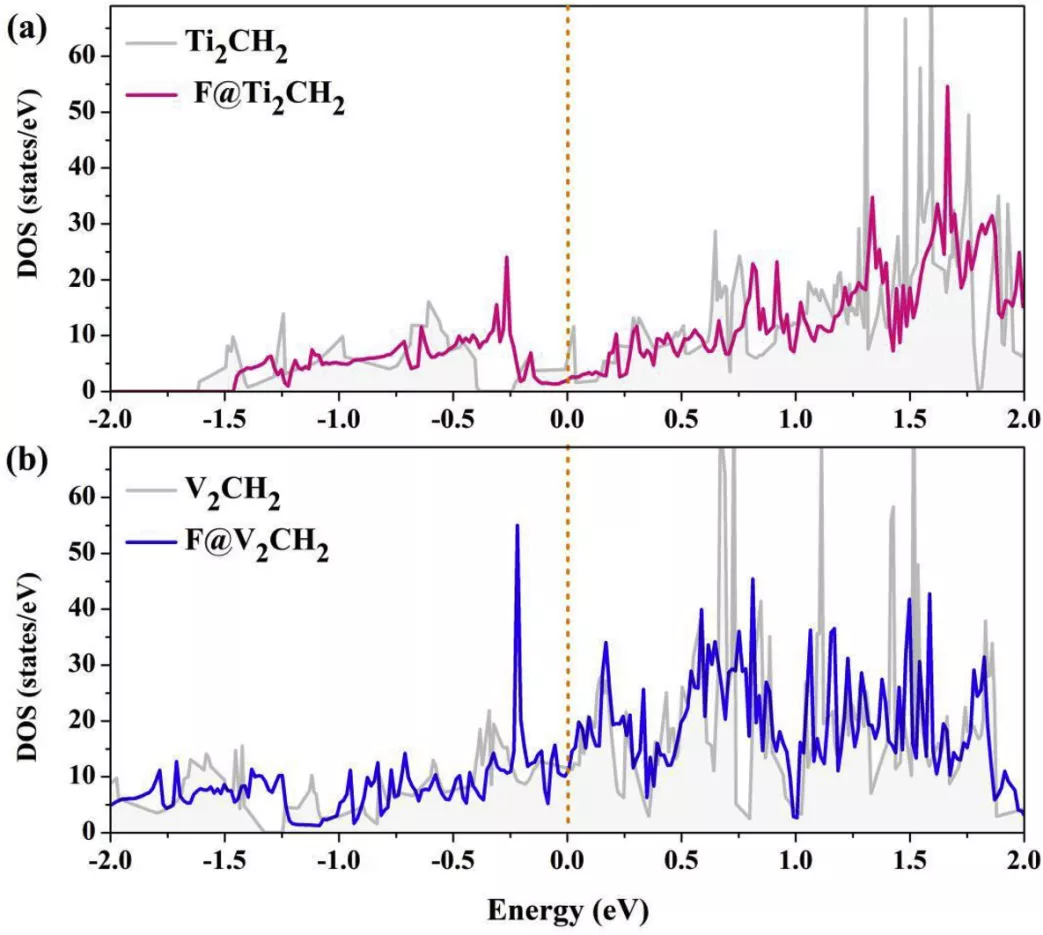
FIG. 6. TDOS with and without the F- and V2CH2 Ti2CH2 monolayer
TDOS of (a) Ti2CH2 and (b ) V2CH2 monolayer with and without F-. the Fermi levels are set to zero and indicated by the dashed lines.

FIG. 7. F- possible migration paths and corresponding energy monolayer Ti2CH2 and V2CH2 barrier
The possible F- migration pathways and the corresponding energy barriers on (a) Ti2CH2 and (b) V2CH2 monolayer, respectively. Schematic representations of the diffusion paths are shown in the insets.
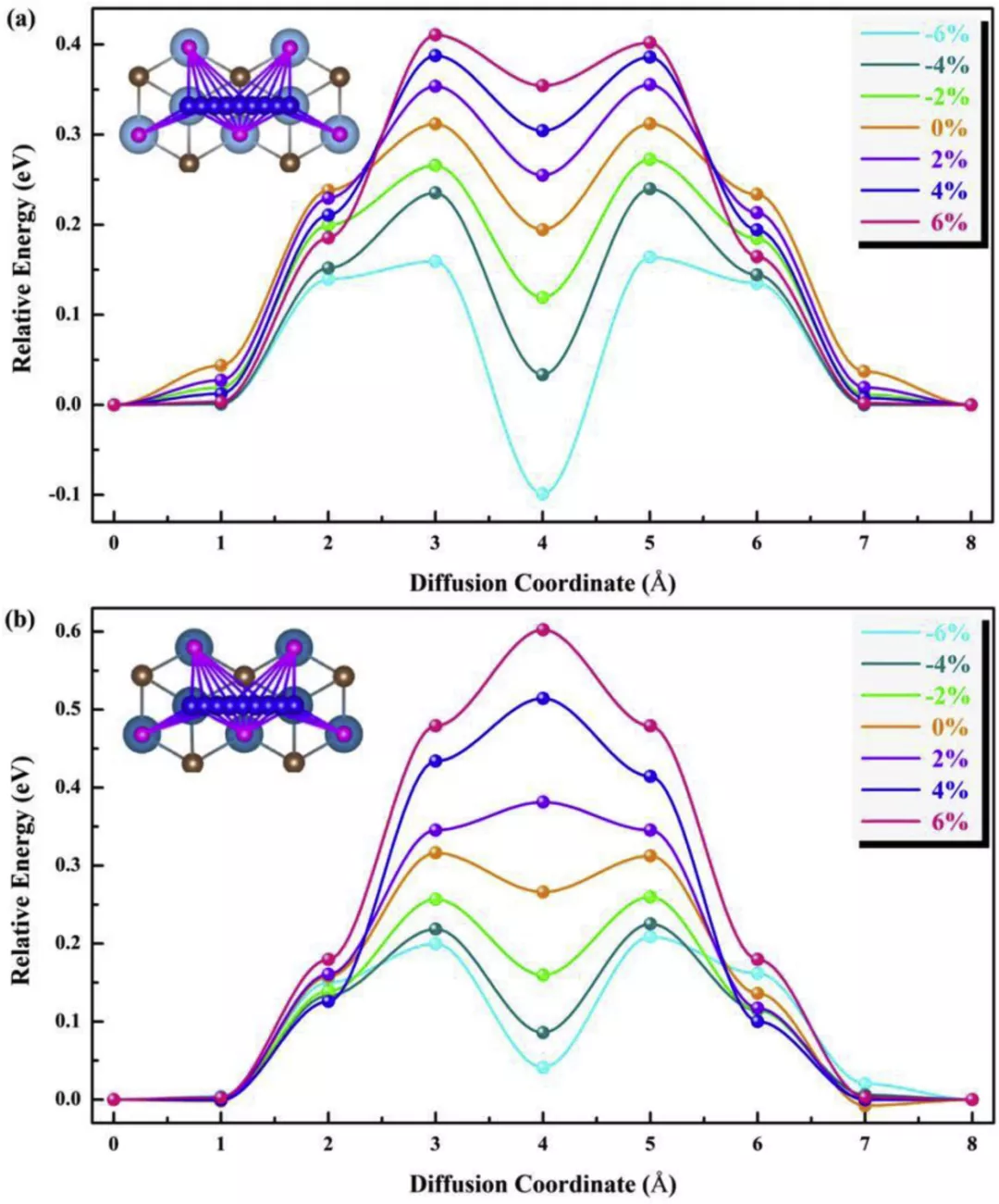
Figure 8.
The potential energy barriers of F-diffusion on (a) Ti2CH2 and (b) V2CH2 monolayer under biaxial strain. Schematic representations of the diffusion paths are shown in the insets
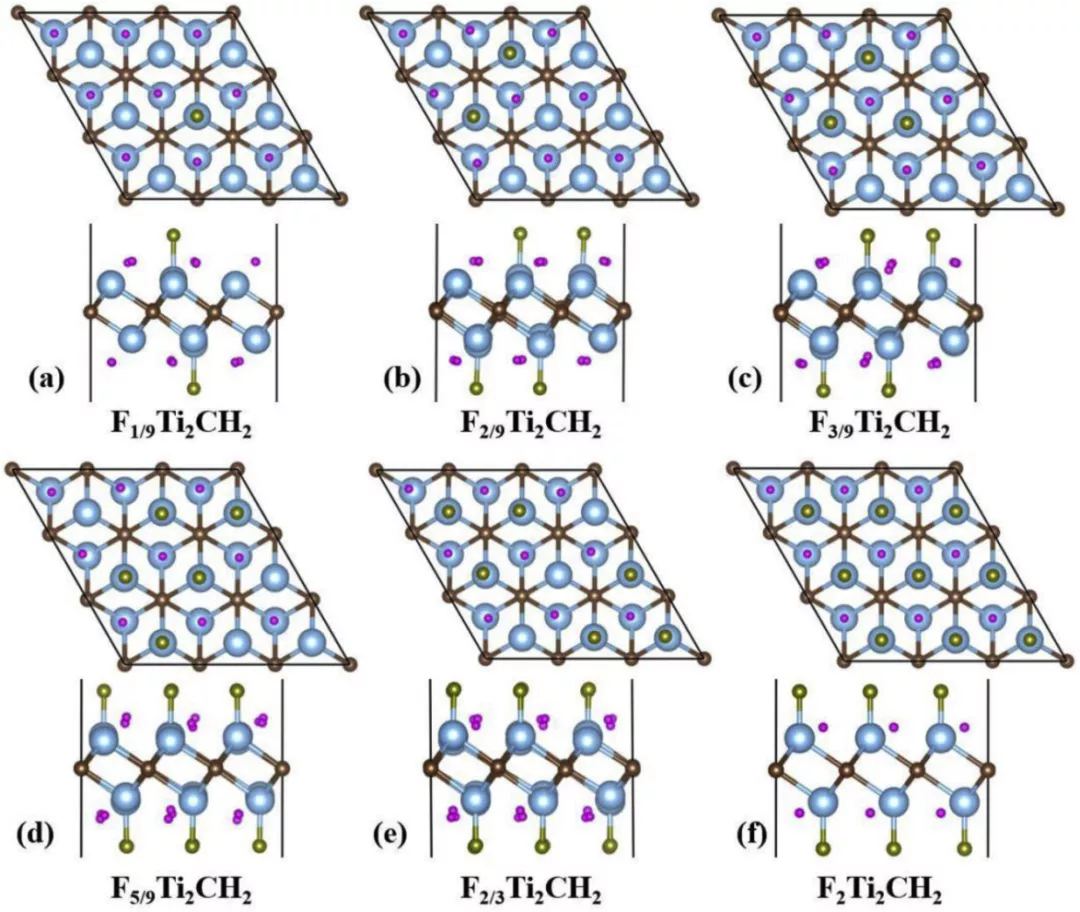
Figure 9.
Top and side views of the optimal structures of FxTi2CH2 with different adsorption concentration: x = 1/9, 2/9, 3/9 , 5/9, 2/3, and 2 (af, respectively).
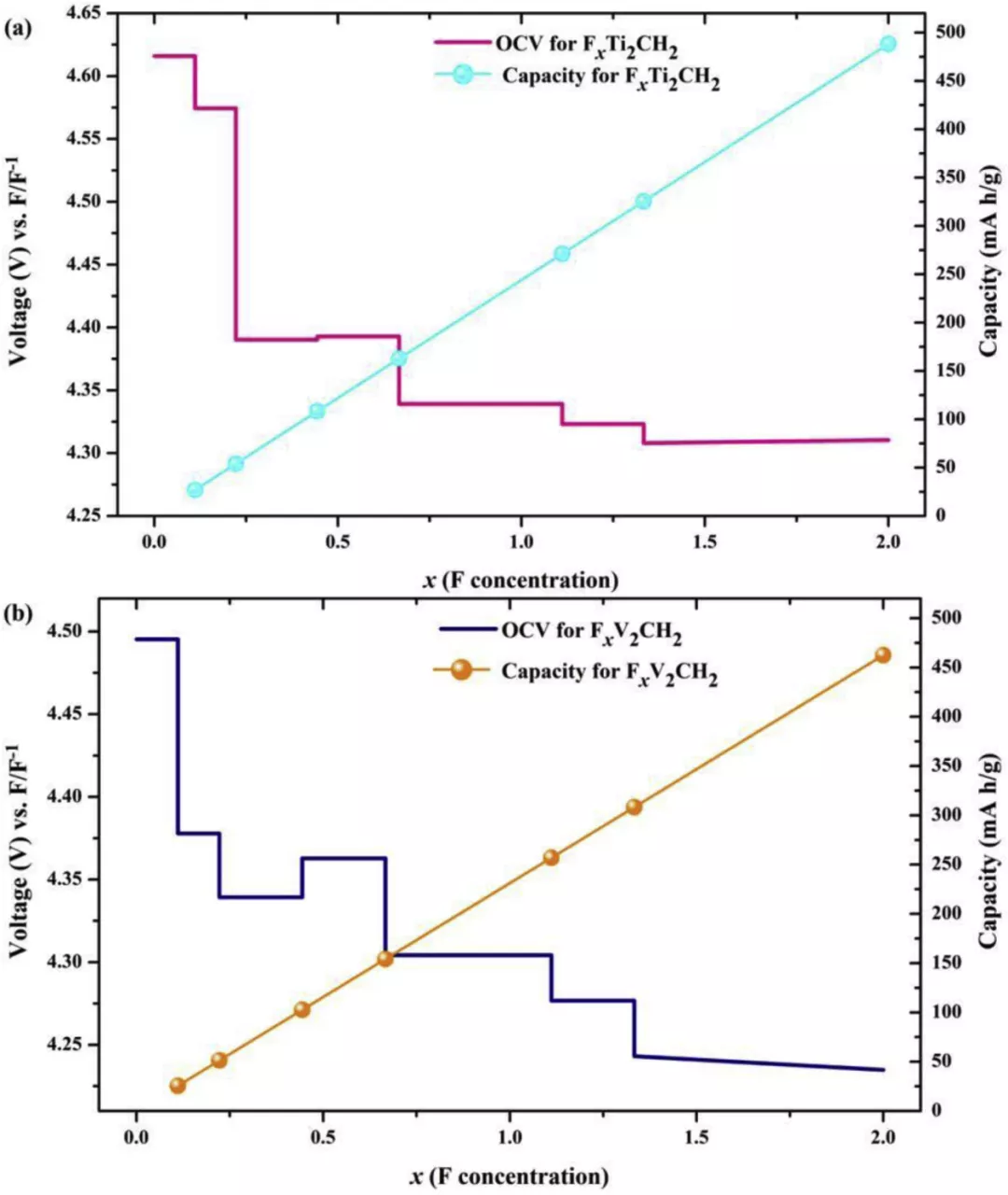
Figure 10. Relationship between Ti2CH2 and V2CH2 average
open circuit voltage (OCV) as the function of F-concentration x in (a) Ti2CH2 and (b) V2CH2 monolayer, respectively.
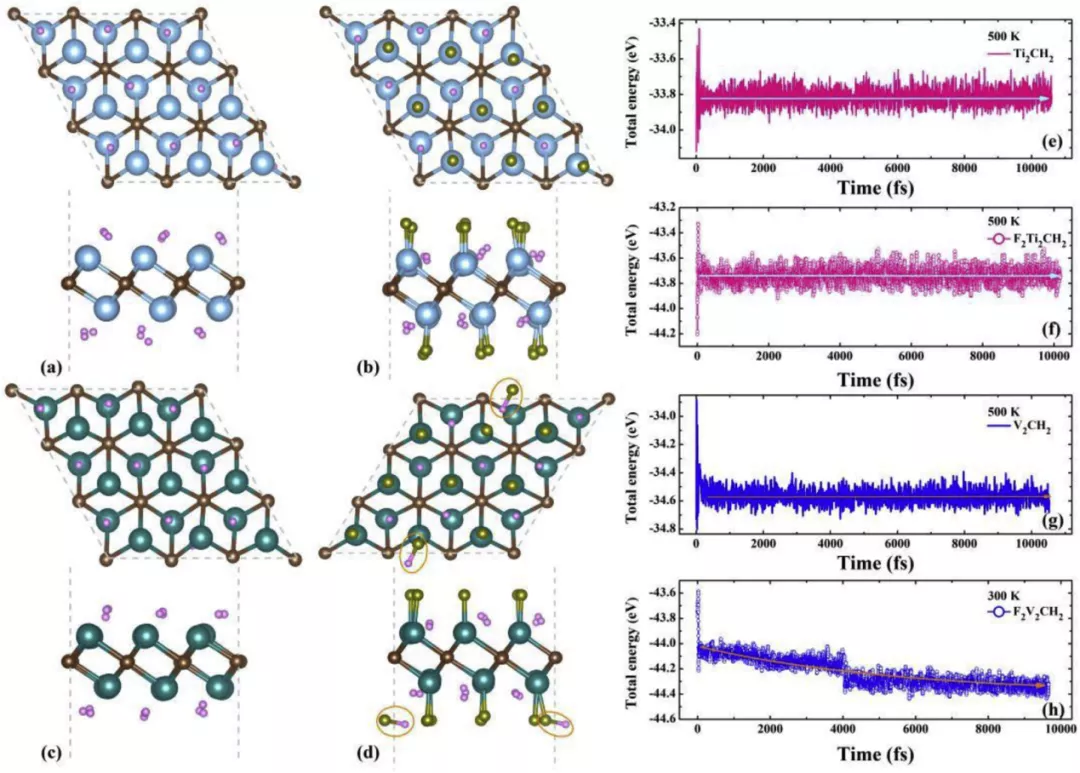
energy stability and FIG 11. Ti2CH2 V2CH2 the
article links:
Fluoride Ion Batteries: Designing flexible M2CH2 (= M of Ti or V) High-Capacity MXenes AS Cathode Materials
https://www.sciencedirect.com/science/article/abs/pii/S2211285520304687
The new concept of rechargeable fluoride ion batteries (FIB) is safer, cheaper and has higher energy density than traditional batteries. However, due to the higher operating temperature (about 340 K) and the need to select electrolytes and electrodes with excellent electrochemical performance, FIB has not been used in daily life. This paper systematically studied several key parameters of M2CH2 (M = Ti or V) MXene to evaluate its performance as a rechargeable FIB cathode material, including energy stability and thermodynamic stability, electronic properties, strain-driven ions Mobility, average open circuit voltage and theoretical specific capacity.
First confirm the ground state structure of M2CH2, and then study the adsorption behavior of F ions on the substrate. Secondly, with and without strain driving, the F micro diffusion path and migration barrier were determined. Theoretical molecular dynamics (AIMD) calculations prove that F2Ti2CH2 is thermodynamically stable at 500 K. With the combination of these key parameters, we prove that the Ti2CH2 monolayer has the potential to be a flexible and strain-controllable cathode material for rechargeable FIB. This article can provide valuable insights for future experiments to design high-performance, dynamic and stable flexible FIB cathode materials.
Keywords
first-principles calculation; fluoride ion battery; M2CH2 (M = Ti or V) MXenes; positive electrode; strain drive; high electrochemical performance
Background introduction
1. MXene is used in lithium-ion batteries The status quo
is found in graphite with excellent performance After ene, different types of two-dimensional (2D) materials have attracted great interest in the fields of materials science and device processing. By introducing 2D layers of transition metal carbides, nitrides and carbonitrides (called MXene, the molecular formula is Mn + 1Xn (where M is a transition metal, X is C and / or N, n = 1, 2 or 3) They have excellent mechanical flexibility, excellent strength and high electrical conductivity. From an experimental point of view, all MXene is produced by etching the MAX phase in concentrated hydrofluoric acid (HF), which causes the surface of MXene to be , O, OH and a small number of F atoms are functionalized.
These terminating groups give MXene a rich surface chemistry and a highly hydrophilic surface, which has been successfully used in batteries, supercapacitors, electrocatalysts for hydrogen release reactions, ion separation membranes, sensors and electromagnetic interference shielding applications. Regarding electrochemical performance, previous studies have shown that MXene can be stably embedded by various monovalent and multivalent cations, resulting in high volume capacitance.
In addition, it has been proved that the types and functional groups of transition metals show a crucial influence on electrochemical performance. As far as lithium batteries are concerned, the capacitance of the additive-free flexible electrode embedded in Ti3C2Tx is much higher than that of graphite, the main negative electrode material. However, lower capacities were obtained in the Nb2CHx and V2CHx layers respectively. In addition, according to the latest report, the relative abundance of lithium on the earth‘s crust is about 20 ppm, which leads to a relatively high production cost associated with lithium. This poses challenges for scientists around the world to actively develop high energy density, safe, cheap and environmentally friendly energy storage equipment.
2. Advantages of fluoride ion battery
Similar to lithium, fluorine has a high electronegativity and a relatively low weight, so it is very stable and has a wide electrochemical stability window.
More importantly, unlike lithium ion batteries (LIB), fluoride ion batteries (FIB) are safer for users due to less overheating and less environmental impact. Fluoride-containing materials have a fluorite-type structure on a global scale, such as CaF2. Therefore, the new concept of FIB has received widespread attention as a promising alternative to LIB.
In theory, the energy density of FIB can reach more than 5000 W h L-1, depending on the electrode structure.
When fluorine reacts with metal to form metal fluoride, a large change in free energy is observed, resulting in a higher theoretical voltage in the electrochemical cell.
3. Research status of FIB and problems to be solved
Although the concept of FIBs is still in the early stages of development, various metal fluorides such as CuF2, PbF2, BiF3, and KBiF4 have been reported in the literature as cathode materials. Batteries with La or Ce metal as the negative electrode, LaF3 or CeF3 doped as the electrolyte, and PbF2 or BiF3 as the positive electrode show a higher capacity. However, it can only work at a fairly high temperature (about 340 K) and is not practical in daily life. To make matters worse, metal anodes (such as Ce, La, Ca, Li or Mg) are very sensitive to surface oxidation, resulting in rapid degradation of the anode. To date, to achieve the effective function of fluoride electrochemical cells, two key challenges should be solved.
One is about choosing the ideal electrolyte for F-transfer, and achieving significant mass transfer efficiency between the electrode and the electrolyte.
Another problem is how to choose suitable electrodes with excellent electrochemical performance, including ion mobility, open circuit voltage, volume capacitance and cycling performance, and excellent mechanical properties such as elastic stiffness, flexibility and strength.
Recently, a liquid electrolyte for fluoride ion conduction has been reported, which indicates that a fluoride conductor at room temperature can be realized, which will also increase interest in developing new electrode materials. Therefore, searching for ideal electrode materials with suitable electrochemical behavior and mechanical properties plays a vital role in the further development of such secondary batteries.
4. Research status of MXene applied to FIB
MXene has proved to be a promising candidate for LIB electrodes. However, to date, there have been few reports about the chemical embedding or large-scale layering of MXene for FIB.

Figure 1. Summary Picture
article describes
based on Nano Energy entitled the above situation, Uppsala University in Sweden Wei Luo and other well-known in the international journal "Fluoride ion batteries: Designing flexible M2CH2 (M = Ti or V) MXenes as high-capacity cathode materials ". Xiao-Yong Yang is the first author of this article.
In this work, the author first studies the electrochemical performance of the representative M2CH2 (M = Ti or V) MXene as the FIBs cathode material. Specifically:
First, the structural stability of M2CH2 with four different configurations is determined by performing first-principle calculations.
Secondly, by analyzing the adsorption energy to determine the most favorable adsorption site.
Then systematically studied the electronic performance and charge compensation mechanism.
In addition, by calculating the migration energy barrier on the possible micro-diffusion path, the F-mobility of the M2CH2 monolayer surface can be predicted. In particular, using the biaxial strain loading scheme (tensile / compression), the F migration energy barrier under various strain states was fully explored. The most important thing is to study the voltage distribution by gradually introducing F on the surface of the M2CH2 monolayer to simulate the F-embedding / de-embedding process and evaluate the corresponding specific energy.
Combining these key electrochemical parameters, some conclusions and suggestions about FIB are drawn.
Article Highlights The
transition metal atom is a key factor in the thermodynamic stability and mechanical flexibility of F2M2CH2 (M = Ti or V) as the electrode material of a fluoride ion battery (FIB).
• In theory, the Ti2CH2 monolayer is a promising cathode material with excellent electrochemical performance of rechargeable FIB.
• The results show that the storage capacity of the Ti2CH2 monolayer in FIB is large (488 (mA h g-1) and the open circuit voltage is high (4.62 V).
• Due to the change in the surface state of the Ti2CH2 monolayer, proper adjustment of the compressive strain can effectively improve F Mobility.

Figure 2. Schematic diagram of M2CH2 structure
Systematic illustration of 1T phase of fully H terminated functionalization M2CH2 (M = Ti or V) MXene system:. Top and side views of (a) MD1, (b) MD2, (c) MD3 and (d) MD4 configuration, respectively

in FIG. 3.
Electronic band structures of (a) Ti2CH2 and (b) V2CH2 in their high-symmetry points. The Fermi level is set to zero.

Figure 4. Schematic diagram
of F-adsorption sites Configures of single F- adsorbed on M2CH2 (M = Ti or V) monolayer.

Figure 5. Schematic diagram of F- adsorbed charge density
Charge density differences (CDD) of single F- adsorbed on TM, TC and TH of Ti2CH2 monolayer (upside) and V2CH2 (downside), respectively. Isosurface level set to 0.003 e Å−3, electron-rich regions, red, and electron -deficient regions, blue. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)

FIG. 6. TDOS with and without the F- and V2CH2 Ti2CH2 monolayer
TDOS of (a) Ti2CH2 and (b ) V2CH2 monolayer with and without F-. the Fermi levels are set to zero and indicated by the dashed lines.

FIG. 7. F- possible migration paths and corresponding energy monolayer Ti2CH2 and V2CH2 barrier
The possible F- migration pathways and the corresponding energy barriers on (a) Ti2CH2 and (b) V2CH2 monolayer, respectively. Schematic representations of the diffusion paths are shown in the insets.

Figure 8.
The potential energy barriers of F-diffusion on (a) Ti2CH2 and (b) V2CH2 monolayer under biaxial strain. Schematic representations of the diffusion paths are shown in the insets

Figure 9.
Top and side views of the optimal structures of FxTi2CH2 with different adsorption concentration: x = 1/9, 2/9, 3/9 , 5/9, 2/3, and 2 (af, respectively).

Figure 10. Relationship between Ti2CH2 and V2CH2 average
open circuit voltage (OCV) as the function of F-concentration x in (a) Ti2CH2 and (b) V2CH2 monolayer, respectively.

energy stability and FIG 11. Ti2CH2 V2CH2 the
article links:
Fluoride Ion Batteries: Designing flexible M2CH2 (= M of Ti or V) High-Capacity MXenes AS Cathode Materials
https://www.sciencedirect.com/science/article/abs/pii/S2211285520304687
Source of information: Scientific Materials Station
- Previous: Dalian Institute of Ch
- Next: MXene breakthrough: Na


 mxene academic
mxene academic
