
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

 With the rise of two-dimensional materials represented by graphene, the research and regulation of the unique electrical properties of two-dimensional materials has also become one of the research hotspots in materials science. Recently, in the field of secondary battery research, in addition to graphene, two-dimensional transition metal sulfide (TMD) materials have also been used as a new type of electrode material in the design and development of lithium-ion batteries. Among them, TiS2, as a very interesting two-dimensional TMD electrode material, has a traditional 1T phase structure in crystal structure, but it does have unique electrical properties. In fact, TiS2 is also the earliest cathode material used in lithium-ion batteries because it has excellent electronic conductivity and ionic conductivity at the same time. The lithium ion diffusion coefficient in TiS2 is about 10-8-10-7cm2/s, which is an order of magnitude higher than traditional oxide cathode materials. A very special recent study found that lithium batteries using TiS2 as the cathode material still retain 50% of the effective charging capacity after storage for 35 years, which verified the long-term stability of the material. In addition, as a sulfide material, TiS2 has a natural good compatibility with potential sulfur-based solid electrolytes. In recent years, TiS2 has not only been used in lithium-ion batteries, but also widely used in sodium ion, potassium ion, and magnesium ion batteries.
With the rise of two-dimensional materials represented by graphene, the research and regulation of the unique electrical properties of two-dimensional materials has also become one of the research hotspots in materials science. Recently, in the field of secondary battery research, in addition to graphene, two-dimensional transition metal sulfide (TMD) materials have also been used as a new type of electrode material in the design and development of lithium-ion batteries. Among them, TiS2, as a very interesting two-dimensional TMD electrode material, has a traditional 1T phase structure in crystal structure, but it does have unique electrical properties. In fact, TiS2 is also the earliest cathode material used in lithium-ion batteries because it has excellent electronic conductivity and ionic conductivity at the same time. The lithium ion diffusion coefficient in TiS2 is about 10-8-10-7cm2/s, which is an order of magnitude higher than traditional oxide cathode materials. A very special recent study found that lithium batteries using TiS2 as the cathode material still retain 50% of the effective charging capacity after storage for 35 years, which verified the long-term stability of the material. In addition, as a sulfide material, TiS2 has a natural good compatibility with potential sulfur-based solid electrolytes. In recent years, TiS2 has not only been used in lithium-ion batteries, but also widely used in sodium ion, potassium ion, and magnesium ion batteries.
An important reason why TiS2 is attractive as an electrode material is its intrinsic high electronic conductivity. Although the electrical properties of TiS2 have been studied for more than half a century, there has been no consensus on the origin of the intrinsically high conductivity of TiS2. Especially in recent years, based on density functional theory calculations and the conductive properties of similar metals, TiS2 is generally considered to be a semi-metallic material. However, other experiments including spectroscopy also believe that TiS2 may be a semiconductor material. These differences in basic understanding will undoubtedly hinder the further optimization of its performance as an electrode material and its development as new functional materials (such as thermoelectric materials, etc.). Recently, a number of research teams from the Chinese Academy of Sciences, the United States, and Singapore have collaborated for three years, using surface characterization measurement technology combined with first-principles calculations to conduct in-depth research on the origin of the intrinsic high conductivity of TiS2. Scanning tunneling microscopy (STM) research found that there are many point defects distributed on the surface of TiS2 (Figure 1). Scanning tunneling spectroscopy (STS) research found that the intrinsic TiS2 electronic structure presents a standard n-type semiconductor feature with no defects on the surface There is a band gap of about 0.5 eV between the valence band and the conduction band. The strictly convergent GW calculation results also verify the existence of the band gap. In the defective position, the STS shows an obvious defect state in the forbidden band. Through first-principles calculations, using the SCAN-rVV10-U method to combine nearly 600 atoms of supercells, the study of point defects in TiS2 (Figure 2) points out that Ti interstitial atoms are defects observed in the experiment. It is also considered to be the main reason why TiS2 is heavily self-doped and causes it to show some metallic properties.
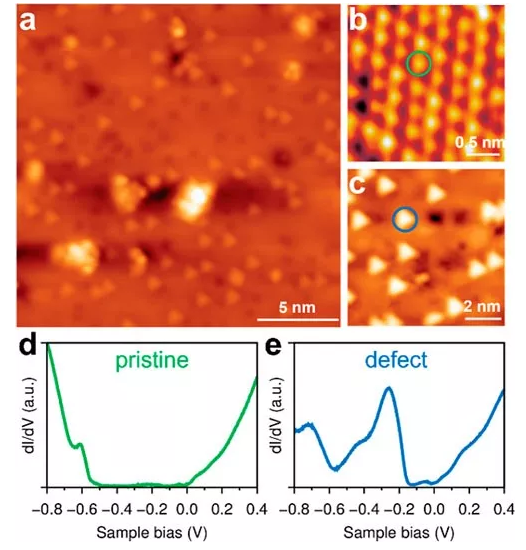
Figure 1. TiS2 surface defects shown in STM image and the corresponding STS spectrum
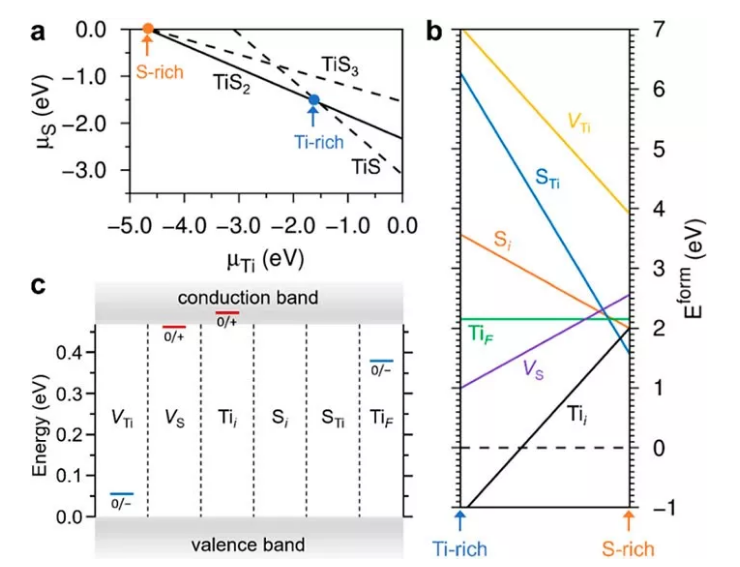
Figure 2. Theoretical calculation of the formation energy, conductivity type and activation energy of various possible defects in TiS2
This result was recently published in J. Phys. Chem. Lett. The co-first authors of the article are Dr. Han Wang from the Lawrence Berkeley Laboratory, PhD student Qiu Zhizhan from the Department of Chemistry, National University of Singapore, and the Department of Physics, State University of New York at Buffalo, USA Doctoral student Xia Weiyi, and the corresponding authors are researcher Xu Hai from Changchun Institute of Optics and Mechanics, Chinese Academy of Sciences and researcher Sun Yiyang from Shanghai Institute of Ceramics.
Original text (scan or long press the QR code, and go directly to the original page after identification): Semimetal or Semiconductor: The Nature of High Intrinsic Electrical Conductivity in TiS2Han Wang, Zhizhan Qiu, Weiyi Xia, Chen Ming, Yuyan Han, Liang Cao, Jiong Lu , Peihong Zhang, Shengbai Zhang, Hai Xu,* Yi-Yang Sun*J. Phys. Chem. Lett., 2019, 10, 6996-7001, DOI: 10.1021/acs.jpclett.9b02710

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |