
First of:. Y.-S Su, E. S. Lamb, I. Liepuoniute
Corresponding author:. Y.-S Su, I. Liepuoniute, S. E. Brown, M. A. Garcia-Garibay
Correspondence unit: University of California, Los Angeles
main content
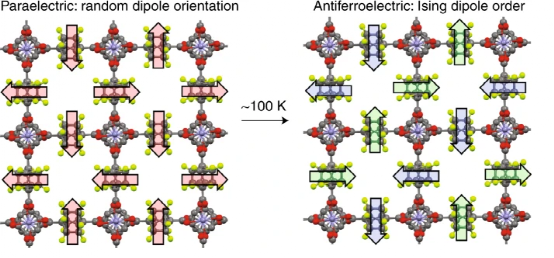 Figure 1. The change in the dipole of the MOF with the rotor structure causes the change in magnetism.
Amphidynamic crystal (Amphidynamic crystal) is a kind of crystal material that can be dynamically changed. In view of this, UCLA Y.-S. Su, I. Liepuoniute, SE Brown, MA Garcia-Garibay, etc. The polar rotor is assembled in the MOF structure material, and it is composed of Zn(II) node and two amphiphilic bicyclic [2.2.2] octane linkers (one of which is assigned to the Zn cluster through the 1,4-aza group On the compound; a modified difluoro derivative through 1,4-dicarboxylic acid coordinated to the Zn cluster compound). During the cooling process, the overall structure of the connecting body follows the dipole-dipole interaction law. When the temperature changes, the frequency-dependent dielectric test shows that the structural transition temperature Tc=100 k. During this transition, the rapidly rotating dipole disordered paraelectric phase transforms into a regular antiferromagnetic crystal phase, in which the dipole moments of the rotor interact with each other. offset. The Monte Carlo simulation of the two-dimensional rotor crystal structure reveals the ground state Ising symmetry, as well as the dipole-lattice and dipole-dipole interactions.
Figure 1. The change in the dipole of the MOF with the rotor structure causes the change in magnetism.
Amphidynamic crystal (Amphidynamic crystal) is a kind of crystal material that can be dynamically changed. In view of this, UCLA Y.-S. Su, I. Liepuoniute, SE Brown, MA Garcia-Garibay, etc. The polar rotor is assembled in the MOF structure material, and it is composed of Zn(II) node and two amphiphilic bicyclic [2.2.2] octane linkers (one of which is assigned to the Zn cluster through the 1,4-aza group On the compound; a modified difluoro derivative through 1,4-dicarboxylic acid coordinated to the Zn cluster compound). During the cooling process, the overall structure of the connecting body follows the dipole-dipole interaction law. When the temperature changes, the frequency-dependent dielectric test shows that the structural transition temperature Tc=100 k. During this transition, the rapidly rotating dipole disordered paraelectric phase transforms into a regular antiferromagnetic crystal phase, in which the dipole moments of the rotor interact with each other. offset. The Monte Carlo simulation of the two-dimensional rotor crystal structure reveals the ground state Ising symmetry, as well as the dipole-lattice and dipole-dipole interactions.
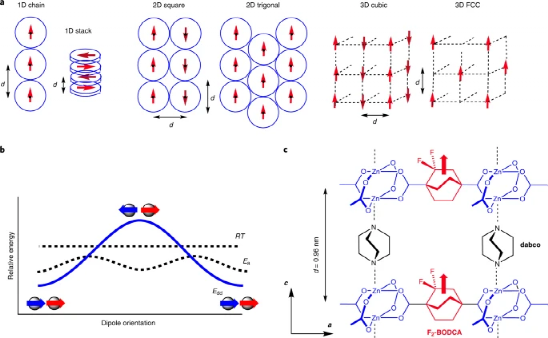 Figure 2. Symmetry breaking leads to the evolution of the dipole lattice.
The thermal motion of the rotor causes the dielectric response of the MOF to change
Figure 2. Symmetry breaking leads to the evolution of the dipole lattice.
The thermal motion of the rotor causes the dielectric response of the MOF to change
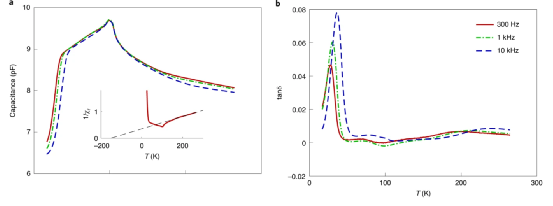 Figure 3. Temperature changes cause complex dielectric response.
In this system, the dipole moment introduced by difluorine atoms is small, so when the temperature reaches 300 K, it is difficult to observe the dipole-dipole interaction. When the rotors rotating barrier potential is low, the dipole-dipole can be observed at low temperatures. Extremely interactive. The low-frequency dielectric test and NMR spectroscopy verify that the rotors dielectric dipole and crystal interact with each other. At higher temperatures, the polar rotor forms a disordered state due to thermodynamics, and the dielectric properties show a Curie-Weiss variation with temperature. At the same time, the dielectric constant exhibits a peak value at T=100 K, which corresponds to the transformation of the crystalline phase to antiferromagnetism. This shows that the value of the rotation potential is not particularly large, which is verified by DFT calculation. Plot the relationship between the capacitance C, the loss tangent tanδ (loss tangent) and the temperature. The results show that the peak value is displayed at ~100 K, and then the capacitance C slowly decreases when the temperature decreases. When the temperature decreases to 35~45 K, the capacitance decays sharply. The change of the loss angle with temperature, which is closely related to the frequency, verifies that this capacitance change is related to the rotation frequency.
Figure 3. Temperature changes cause complex dielectric response.
In this system, the dipole moment introduced by difluorine atoms is small, so when the temperature reaches 300 K, it is difficult to observe the dipole-dipole interaction. When the rotors rotating barrier potential is low, the dipole-dipole can be observed at low temperatures. Extremely interactive. The low-frequency dielectric test and NMR spectroscopy verify that the rotors dielectric dipole and crystal interact with each other. At higher temperatures, the polar rotor forms a disordered state due to thermodynamics, and the dielectric properties show a Curie-Weiss variation with temperature. At the same time, the dielectric constant exhibits a peak value at T=100 K, which corresponds to the transformation of the crystalline phase to antiferromagnetism. This shows that the value of the rotation potential is not particularly large, which is verified by DFT calculation. Plot the relationship between the capacitance C, the loss tangent tanδ (loss tangent) and the temperature. The results show that the peak value is displayed at ~100 K, and then the capacitance C slowly decreases when the temperature decreases. When the temperature decreases to 35~45 K, the capacitance decays sharply. The change of the loss angle with temperature, which is closely related to the frequency, verifies that this capacitance change is related to the rotation frequency.
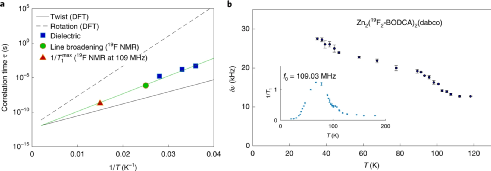 Figure 4. Dielectric characterization in molecular dynamics and the results of NMR measurement with temperature changes.
The frequency change rule in the 35~45 K interval was investigated. The change process in this interval was caused by the activation energy barrier in the Debye-like relaxation model. By testing the system, the dipole momentum and dipole-dipole interaction energy can be obtained from the experimental results.
Thermodynamic change mechanism
The DFT calculation method is used to calculate and analyze the energy barrier that activates the relaxation motion, which is used to analyze the interaction between the rotors and the rotor dynamics. The calculation results show that the energy barriers for twisting and rotating separately are 0.8 kcal mol-1 and 2.0 kcal mol-1, the absolute confidence interval is 0.2~0.4 kcal mol-1, and the coupled rotation-torsion energy barrier is 1.4 kcal mol -1.
For the MOF molecular system with unmodified fluorine atoms, the double symmetry of the carboxylic acid group and the triple symmetry of the rotor form a rotor with sixfold symmetry in the MOF. The symmetry of the MOF system modified with fluorine atoms changes, and the barrier potential is generated between the conversion between the C1+ and C1- conformations. Through this conformational change, the order-disorder state change is realized, but the dielectric change and Debye solidification in the ordered structure are caused by the dipole oscillation.
The author uses classic Monte Carlo calculations and simulations, and simulates the electronic dipole interaction during the paraelectric-antiferroelectric crystal phase change process through a system with similar or higher energy. In order to simplify the calculation model, the author only divides adjacent and spaced adjacent The interaction of the two dipoles is investigated during the simulation calculation.
In addition, the relationship between the rotational potential energy and the rotor-lattice interaction is also investigated, which explains the frequency-dependent Debye dynamics found in experimental observations. Among them, the rigidity of the lattice potential is thermally activated to cause the rotor torsion to become disordered. When the thermally activated torsion effect is eliminated, the rotation is inhibited, and the dielectric response is affected.
references
Su, YS., Lamb, E.S., Liepuoniute, I. et al. Dipolar order in an amphidynamic crystalline metal--organic framework through reorienting linkers, Nat. Chem. (2021).
DOI: 10.1038/s41557-020-00618-6
https://www.nature.com/articles/s41557-020-00618-6
Figure 4. Dielectric characterization in molecular dynamics and the results of NMR measurement with temperature changes.
The frequency change rule in the 35~45 K interval was investigated. The change process in this interval was caused by the activation energy barrier in the Debye-like relaxation model. By testing the system, the dipole momentum and dipole-dipole interaction energy can be obtained from the experimental results.
Thermodynamic change mechanism
The DFT calculation method is used to calculate and analyze the energy barrier that activates the relaxation motion, which is used to analyze the interaction between the rotors and the rotor dynamics. The calculation results show that the energy barriers for twisting and rotating separately are 0.8 kcal mol-1 and 2.0 kcal mol-1, the absolute confidence interval is 0.2~0.4 kcal mol-1, and the coupled rotation-torsion energy barrier is 1.4 kcal mol -1.
For the MOF molecular system with unmodified fluorine atoms, the double symmetry of the carboxylic acid group and the triple symmetry of the rotor form a rotor with sixfold symmetry in the MOF. The symmetry of the MOF system modified with fluorine atoms changes, and the barrier potential is generated between the conversion between the C1+ and C1- conformations. Through this conformational change, the order-disorder state change is realized, but the dielectric change and Debye solidification in the ordered structure are caused by the dipole oscillation.
The author uses classic Monte Carlo calculations and simulations, and simulates the electronic dipole interaction during the paraelectric-antiferroelectric crystal phase change process through a system with similar or higher energy. In order to simplify the calculation model, the author only divides adjacent and spaced adjacent The interaction of the two dipoles is investigated during the simulation calculation.
In addition, the relationship between the rotational potential energy and the rotor-lattice interaction is also investigated, which explains the frequency-dependent Debye dynamics found in experimental observations. Among them, the rigidity of the lattice potential is thermally activated to cause the rotor torsion to become disordered. When the thermally activated torsion effect is eliminated, the rotation is inhibited, and the dielectric response is affected.
references
Su, YS., Lamb, E.S., Liepuoniute, I. et al. Dipolar order in an amphidynamic crystalline metal--organic framework through reorienting linkers, Nat. Chem. (2021).
DOI: 10.1038/s41557-020-00618-6
https://www.nature.com/articles/s41557-020-00618-6







