
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

Inorganic solid electrolytes for lithium-ion batteries are expected to make breakthroughs in improving safety and energy density. In particular, the mechanically deformed sulfide solid electrolyte (SE) material can be integrated into the composite electrode through a simple cold pressing process, thereby avoiding the error-prone high-temperature sintering process. In addition, the Li+ conductivity of these most advanced candidate materials has reached 10ms cm-1, which is equivalent to traditional organic liquid electrolytes. Hybridization with polymer binders for actual ASLB is imperative because of their positive effect of releasing mechanical stress in the prepared large cells, as well as fast ion transport channels. However, sulfides are susceptible to the nucleophilic attack of the polar functional groups of common organic solvents, which severely limits the available slurry processing solvents to only non-polar or low-polar solvents. Their chemical sensitivity to common organic solvents such as N-methylpyrrolidone (NMP) has brought insurmountable obstacles to the industrialized mass production of all-solid-state batteries.
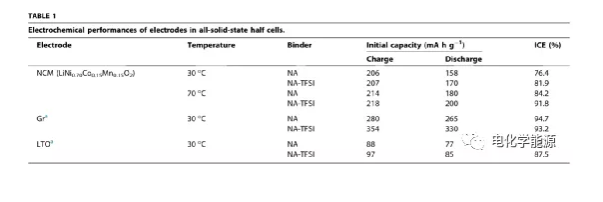
Recently, Yoon Seok Jung of Yonsei University in South Korea, Sungeun Jeoung of the National Ulsan Institute of Science and Technology, etc. reported a new tactical method to combine sulfide SES with thermal stability and slurry-based non-liquid dry polymer electrolyte (DPE). Lithium ion conductive adhesive (DPE) type adhesive is hybridized. In addition to practicality, ester solvents containing a large amount of hydrocarbons (such as benzyl acetate) can dissolve polymers and lithium salts (such as LiTFSI) without causing damage to sulfides. Using DPE type binder NA-LiTFSI (NA: nitrile rubber-poly(1,4-butylene succinate)) in LiNi0.70Co0.15Mn0.15O2(NCM) electrode can significantly increase its 30℃ Under the electrochemical performance. In addition, NA-LiTFSI has strong functionality at 70°C (180-200 mA·h-1, initial coulombic efficiency of 84.2-91.8%) and is also suitable for other electrodes, such as graphite. Finally, a soft pack battery NCM/graphite all-solid-state battery was prepared with electrodes made of NA-LiTFSI binder, which showed good performance.
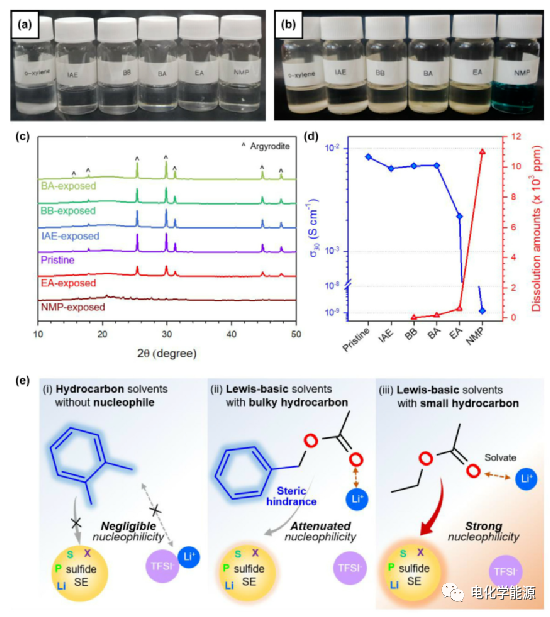
The results of the compatibility of the slurry processing solvent with sulfide and lithium salt respectively.
(A) A photograph of a mixture of LiTFSI and a solvent, and (B) a photograph of a mixture of LPSX and a solvent.
(C) X-ray diffraction patterns of LPSX in different solvents of NMP (N-methylpyrrolidone), EA (ethyl acetate), BA (benzyl acetate), BB (butyl butyrate) and IAE (isoamyl ether) .
(D) The Li+ conductivity of LPSX at 30°C and the amount of dissolution after exposure to solvents.
(E) Hydrocarbon solvents (such as o-xylene), Lewis-basic solvents containing large volume of hydrocarbons (such as BA), Lewis-basic solvents containing small hydrocarbons (such as EA) and the reactivity of LPSX . Lewis-alkaline solvents containing large volumes of hydrocarbons, such as BA. It does not react with LPSX due to the steric hindrance of the macromolecular benzyl group, and can dissolve the lithium salt due to the polar acetate group.
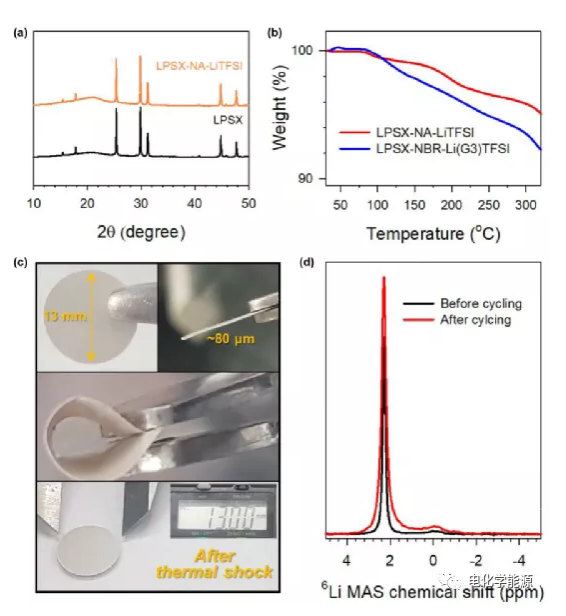
Characterization of LPSX-NA-LiTFSi composite material.
(A) XRD patterns of original LPSX and LPSX-NA-LiTFSi.
(B) Thermogravimetric analysis (TGA) curves of SIL type LPSX-NbR-Li(G3)TFSI and liquid-free LPSX-NA-LiTFSI in Ar.
(C) Photograph of flexible and thermally stable LPSX-NA-LiTFSI film (intact after thermal shock at 200°C for 1 hour).
(D) MAS6Li NMR spectra of LPSX-NA-LiTFSi before and after 6Li/(LPSX-NA-LiTFSi)/6Li symmetric battery cycle.
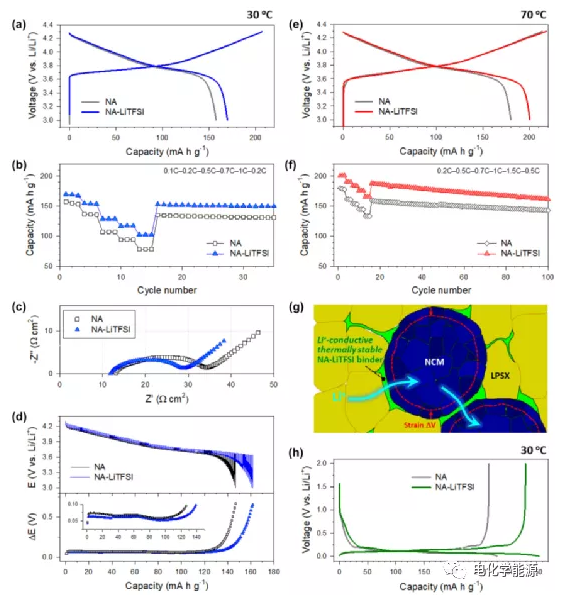
Electrochemical characterization of NCM and Gr in all solid-state half-cells
(A) 0.1C first charge and discharge voltage curve
(B) Rate capacity
(C) Nyquist curve
(D) Transient discharge voltage distributions of NCM electrodes without LiTFSi and LiTFSi binders obtained from GITT and their corresponding polarization curves (NA and NA-LiTFSI, respectively).
(E) The first week charge and discharge voltage distribution at 0.2C and (F) the rate capability of the LiTFSI-free and LiTFSI-binder-made NCM electrodes made of binder.
(G) The microstructure of the NCM composite electrode made of DPE type binder (such as NA-LiTFSi). Although the electrode active material of NCM has volume strain, these binders provide a smooth interface ion transmission path, and No thermal degradation will occur. (H) The first cycle charge-discharge voltage distribution of LiTFSI-free and LiTFSI-containing Gr electrodes made of a binder at 0.1C and 30C.
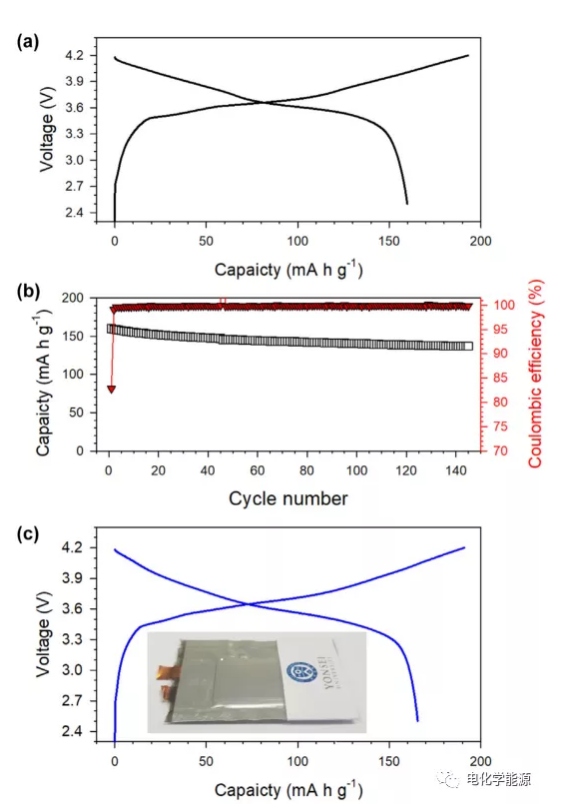
The test results of NCM/Gr all-solid-state battery at 30°C (A) the first cycle charge and discharge voltage distribution and (B) the cycle performance of the spherical full battery. (C) The first cycle charge and discharge voltage distribution of the full pouch battery.
to sum up
A new tactical mixing solution was developed to prepare a slurry-like Li+ conductive DPE adhesive with high thermal stability. Ester solvents containing a large amount of alkyl groups (such as BA (benzyl acetate)) have practical adaptability as a slurry solvent (non-toxic and have appropriate vapor pressure), can dissolve polymers and lithium salts, and are vulnerable The sulfide remains intact when in contact. Using BA and DPE type adhesives, a wet slurry method was used to prepare flexible SE films and sheet electrodes with good thermal stability. The use of DPE-type binders, such as NA-LiTFSI, significantly improves the utilization of NCM (158~170 mAh/g) and Gr (265~320 mA h/g) electrode active materials. In addition, the DPE type adhesive maintains its functionality even under high temperature test conditions of 70°C. These results are consistent with all-solid-state batteries using SIL-type binders (such as NBR-Li(G3)TFSi) for Gr electrodes or all-solid-state batteries under thermal abuse conditions (thermal shock before testing or at 70°C) The poor electrochemical performance is a sharp contrast. In addition, the feasibility of the DPE binder composed of polymers such as PVA and PPC was also confirmed, thus highlighting the wide applicability of the developed strategy. Finally, the NCM/Gr all-solid-state battery using electrodes and the SE film made of Na-LiTFSI showed good performance. The research results clarified the design strategy of composite materials and marked a breakthrough in the development of practical all-solid-state technology.
Article information
Tactical hybrids of Li+-conductive dry polymer electrolytes with sulfide solid electrolytes: Toward practical all-solid-state batteries with wider temperature operability
Materials Today (IF 26.416) Pub Date: 2021-02-27, DOI: 10.1016/j.mattod.2021.01.006
Dae Yang Oh; Kyu Tae Kim; Sung Hoo Jung; Dong Hyeon Kim; Seunggoo Jun; Sungeun Jeoung; Hoi Ri Moon;

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |