
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

Background.
Transition metal phosphate has been widely reported because of its high theoretical specific capacitance, high natural abundance and environmental and biological harmlessness, so it is one of the ideal electrode materials for supercapacitors. In addition, because of the strong PmurO tetrahedron in its structure, transition metal phosphates show excellent stability and applicability. Although some previous works have proved the advantages of transition metal phosphates, they still have some problems, such as limited porosity, low electrochemical specific surface area and poor conductivity, resulting in slow electrochemical reaction kinetics. Therefore, it is very important to develop a new synthesis strategy of transition metal phosphate electrode.
Full text guide.
In view of this, Ni-MOF amorphous nanospheres were self-assembled on MXene nanowires, and then MOF nanospheres were converted into graded porous nickel phosphate nanospheres by etching reaction, and MXene-NPO materials were prepared. Porous nickel phosphate nanospheres are fixed on MXene nanowires, which enables rapid electron transfer between nickel phosphate and MXene, and solves the problem of low electrical conductivity of nickel phosphate. At the same time, porous nickel phosphate nanospheres can prevent the aggregation of MXene nanowires and maintain the layered structure, which is conducive to the rapid ion migration in the electrode. The electrode material exhibits a high specific capacity of 639 C g murl 1 at 0.5 A g-1 and has excellent cycle stability of 85% capacity retention after 10000 cycles. The asymmetric supercapacitor prepared by using MXene-NPO as negative electrode and p-phenylenediamine functionalized reduction of graphene oxide (PPD-rGO) as positive electrode exhibits a considerable energy density of 72.6W / h kg-1 at a power density of 932W / kg-1. Therefore, the design strategy has important guiding significance for the preparation of high-performance electrode materials. The results of related papers are published on Chemical Engineering Journal under the title "Self-assembly of MOF on MXene nanosheets and in-situ conversion into superior nickel phosphates/MXene battery-type electrode".
Preparation of nickel phosphate derived from amorphous MOFs modified in situ on MXene. Firstly, MXene nanowires were obtained by etching Ti3AlC2 powder in HF aqueous solution. The prepared MXene nanowires provide rich interlayer functional groups and wide surfaces to accelerate the in-situ growth of amorphous MOF and obtain MXene-MOF. Then, using KH2PO4 as phosphorus source, MXene-MOF was hydrothermally treated to achieve in-situ phosphorylation of MOF, and MOF spheres were etched into graded porous nickel phosphate nanospheres to obtain MXene-NPO.
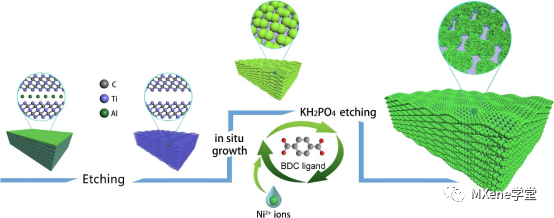
Figure 1 shows:
Schematic diagram of the synthetic route of MXene-NPO.
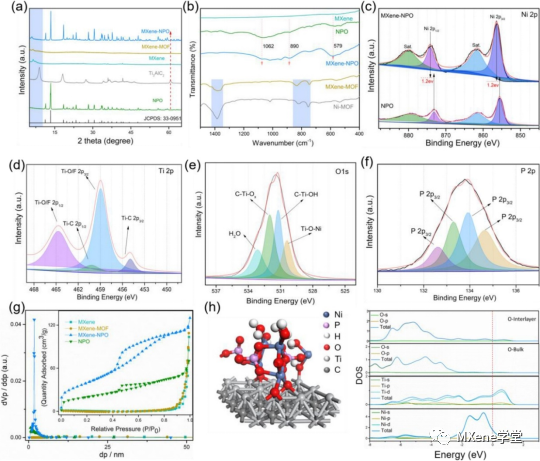
Figure 2 shows:
A) PXRD diagram; b) FTIR spectrogram; Cmurf) the high resolution XPS spectra of Ni2p, Ti2p, O1s and P2p, respectively; g) N2 adsorption / desorption isotherms and pore size distribution curves; h) for the calculation of the MXene-NPO model and the DOS of different atoms.
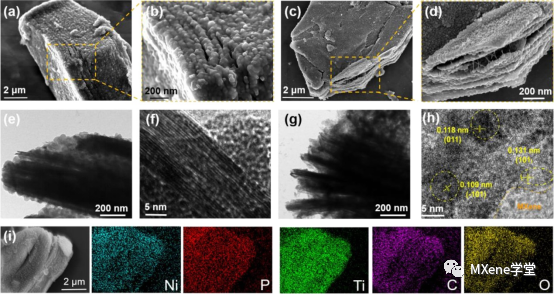
Figure 3:
The morphological characteristics of MXene-MOF and MXene-NPO samples, the TEM images of MXene-MOF, and the element distribution maps of TEM and HRTEM images of MXene-NPO.
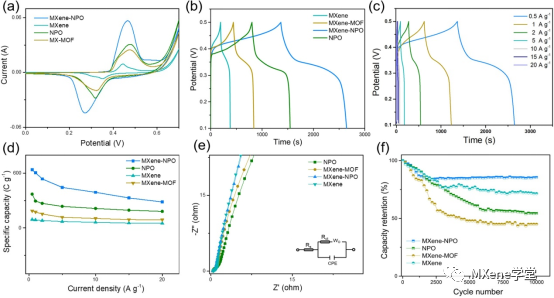
Figure 4 shows:
A) the CV curves of several kinds of electrode materials prepared under 5mv Smurl are compared with the GCD curves of; b) electrode materials at 0.5A / gMuel 1. The GCD curves of; c) MXene-NPO electrodes at different current densities are compared with the GCD curves of several electrodes prepared under different current densities. The specific capacity comparison; e) Nyquist diagram calculated based on the GCD curve (the illustration shows the long cycle performance of the electrode prepared by the equivalent circuit); f) at 10 A / g murl 1).
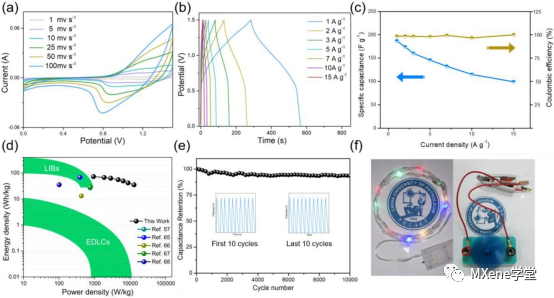
Figure 5 shows:
A) CV curves of MXene-NPO//rGO asymmetric supercapacitors at different scanning rates GCD curves of; c) MXene-NPO//rGO asymmetric supercapacitors at different current densities; d) MXene-NPO//rGO asymmetric supercapacitors compared with the previously reported results Ragone diagram of; e) MXene-NPO//rGO capacitors cycle performance of; f) MXene-NPO//rGO capacitors at 5 A g Mel 1 physical diagram of; f) MXene-NPO//rGO capacitors.
Summary.
By using the abundant electronegative active sites on MXene to promote the nucleation and growth of MOF, and then in situ converted into porous nickel phosphate, advanced MXene-NPO electrode materials were successfully prepared. In the process of electrochemical reaction, MXene-NPO promotes the electron transfer between nickel phosphate and MXene. At the same time, porous nickel phosphate nanospheres are fixed on MXene nanowires to prevent their aggregation, which helps to maintain the layered structure and make ions migrate rapidly in the electrode, thus realizing a long charge-discharge cycle. The electrode material shows a remarkable specific capacity of 639C g / ml and excellent stability after 10000 cycles at 0.5 A g ~ (- 1). The asymmetric supercapacitor manufactured by using MXene-NPO as negative electrode and PPD-rGO as positive electrode shows a considerable energy density of 72.6W / h kg-1 at a power density of 932W kg-1, and has excellent cycle stability of up to 94% capacity retention after 10000 cycles. This work provides a new strategy for the preparation of high performance electrode materials with great potential.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |