
hotline£º
17715390137
Tel/Wechat£º
18101240246 (Technology)
0512-68565571
Email£ºmxenes@163.com £¨Sales Engineer£©bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

The advantages of supercapacitors and sodium / lithium ion batteries are organically combined by the internal intersection of supercapacitors and sodium / lithium ion battery mechanisms. the construction of sodium / lithium ion hybrid capacitors has caused extensive research and development.This kind of devices can provide an effective solution to the problem of social energy demand with high energy and high power.However, due to the large radius of sodium ions (1.02), the slow diffusion kinetics of sodium ions in the process of charge and discharge is troubled at present. Therefore, the key is to controllably prepare new high-performance electrode materials with multi-stage structure and a large number of active sites.
The highlight of this article.
1. A novel N-doping strategy is proposed to realize the trimerization of C2N3-in the two-dimensional MXene intermediate layer.
two¡£. The ultra-fast pseudo-capacitance behavior of Ti3C2Tx/Na3TCM composite anode is improved and proved.
3. A sodium ion capacitor with high energy density and long cycle life was fabricated by anode / cathode mass matching.
Content introduction.
Ti3C2TxMXene has good metal conductivity and its unique layered structure can store Na+ ions well, so it is a very potential electrode material for sodium ion battery. At present, how to make effective use of a large number of redox active sites on its surface is the key. In order to further improve the sodium storage performance of Ti3C2Tx, Academician Li Xigao of Dalian University of Technology proposed a new strategy to realize cyanide in-situ trimerization between Ti3C2Tx MXene layers, so as to prepare high performance anode materials for sodium ion capacitors. The expanded interlayer spacing and active surface area provide sufficient space for Na+ ions, which is beneficial to improve the structural stability of the materials; on the other hand, the in-situ tripolymerization products replace the-F site and effectively bond with Ti through chemical bonding to achieve a high content of stable N doping and improve the electrochemical reaction kinetics.
The results show that the Ti3C2Tx/Na3TCM material has excellent electrochemical performance. After 1000 cycles at 100 mA/g current density, the reversible capacity can reach 182.2 mAh/g;. The assembled sodium ion capacitor has high energy density (97.6 Wh/kg), power density (16.5 kW/kg) and good long cycle characteristics.
Guided reading of picture and text.
I. Preparation of Ti3C2Tx/Na3TCM composite anode.
As shown in figure 1A, the one-step hydrothermal synthesis strategy is used to realize the in-situ trimerization of C2N3-anions on the interlayer and surface of two-dimensional Ti3C2TxMXene. The oligomer C6N93-replaces the site of-F by stable chemical bonds during the hydrothermal process and exists stably and uniformly in the intermediate layer of Ti3C2Tx. From the FTIR spectrum and XRD spectrum, it can be seen that the structure of Ti3C2Tx has not been destroyed, and the amorphous tripolycarbon product with high N doping is formed. In addition, due to the synergistic effect of trimeric products and free sodium ions, the interlayer spacing of Ti3C2Tx is enlarged from 10.0 to 12.6, which provides an open space for the transport of electrolyte ions and thus alleviates the accumulation of two-dimensional nanowires.
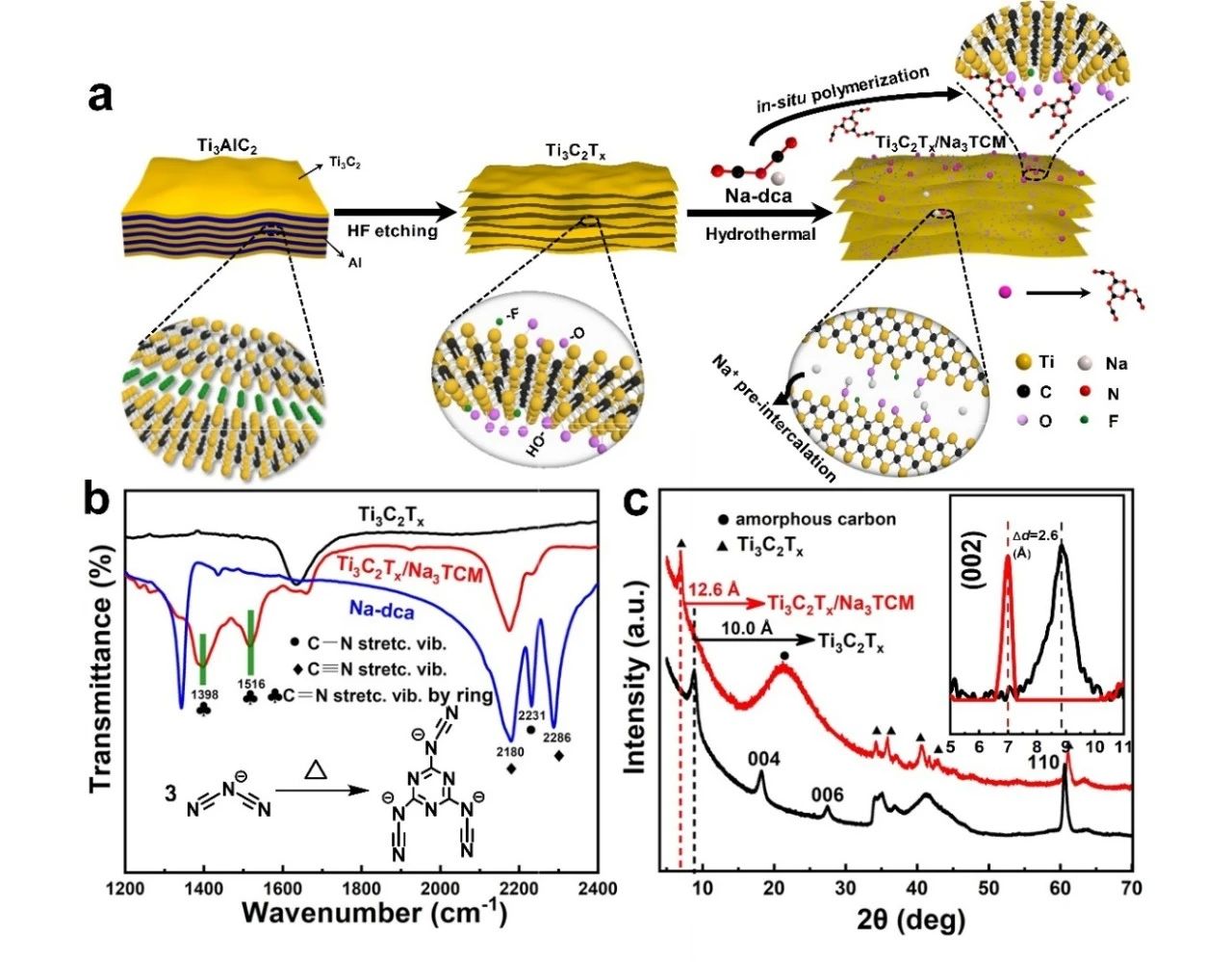
Figure 1. (a) A schematic diagram of the synthesis of Ti3C2Tx/Na3TCM. (B) FTIR spectra of Na-dca,Ti3C2Tx and Ti3C2Tx/Na3TCM, illustrations showing the thermal trimerization process from C2N3-to C6N93 -. (C) XRD maps of Ti3C2Tx and Ti3C2Tx/Na3TCM.
II. Structural characterization of Ti3C2Tx/Na3TCM.
From the high-resolution XPS and Ar ion sputtering images of Ti3C2Tx/Na3TCM and Ti3C2Tx, it can be seen that the oligomer C6N93-binds closely to the exposed Ti atoms on the surface of Ti3C2Tx through chemical bonds. In addition, the content of F atoms decreased from 15.5 to 5.2 at.%,. On the contrary, the content of N atoms increased from 0 to 5.6 at.%,. Nearly 80% of NMel 5 and NMel 6 in triazine rings can quickly store sodium ions and provide high pseudocapacitance. According to the micrograph, Ti3C2Tx/Na3TCM showed an undestroyed stack structure of 2D nanosheets, and the interlayer spacing was expanded. The EDS element distribution map shows that C, Ti, O, N and Na elements are uniformly distributed in Ti3C2Tx/Na3TCM hybrid materials.

Figure 2. (a) High resolution Ti 2p XPS spectra of Ti3C2Tx/Na3TCM and Ti3C2Tx. (B) High resolution N 1s XPS spectra of Ti3C2Tx/Na3TCM and Ti3C2Tx. (C) nitrogen adsorption-desorption isotherms and pore size distributions of Ti3C2Tx/Na3TCM and Ti3C2Tx. (d) SEM diagram of Ti3C2Tx/Na3TCM. (e) HR-TEM images of Ti3C2Tx and (f) Ti3C2Tx/Na3TCM. The (g) STEM images of Ti3C2Tx/Na3TCM and the corresponding element mapping images of h C Magi Ti,j O Magi k N and l Na.
III.Study on Sodium Storage performance of Ti3C2Tx/Na3TCM.
The button battery was assembled with Ti3C2Tx/Na3TCM as the active electrode, sodium as the counter electrode, glass fiber as the separator and 1 M NaClO4 as the electrolyte. The electrochemical performance was tested. The results are shown in figure 3. Ti3C2Tx/Na3TCM showed excellent cycle performance and rate performance, and the capacity did not decay after 1000 cycles at a current density of 100 mA/g. Under the current density of 0.05 mAh/g, 0.1, 0.2, 0.5 and 5 A, its capacity could reach 210,174,157,147 mAh/g, respectively. In addition, the kinetic analysis shows that Ti3C2Tx/Na3TCM exhibits ultra-fast sodium ion storage behavior as a high pseudo-capacitance material.

Figure 3. (a) CV curves of Ti3C2Tx/Na3TCM at different scanning rates. (B) charge-discharge curves after the first cycle and the 20th cycle at a current density of 20 mA/g. (C) the rate performance of the electrode. (d) long-term cycle performance and Coulomb efficiency of the electrode. (e) Nyquist diagram of Ti3C2Tx/Na3TCM and Ti3C2Tx before and after the cycle. (F) the b value of the electrode at the cathode and anode peaks. (G) the b value of the anodic peak of the sample at different potentials. (h) Capacitance contribution at various scanning rates from 0.1to 1 mV/s.
IV. First-principles calculation of Ti3C2Tx/Na3TCM
The feasibility of static adsorption and dynamic intercalation of Na+ ions in Ti3C2Tx/Na3TCM is theoretically calculated by using the first principle, as shown in figure 4. The results show that the Eads (- 3.22 eV) of the Na atom at the most stable site of Ti3C2Tx/Na3TCM is lower than that of the original Ti3C2Tx (+ 3.05 eV). It is easy to show that the increase of N atom reduces the adsorption energy of the whole system, thus promoting the storage of Na ions. In addition, compared with the diffusion on the Ti3C2Tx surface, the migration energy barrier of Na+ ions on the Ti3C2Tx/Na3TCM surface is lower. The calculated results show that the diffusion rate of Na+ ions increases after N doping, and then the surface of Ti3C2Tx/Na3TCM absorbs Na ions more easily, which promotes the extra intercalation of free Na ions in the electrolyte, which is in good agreement with the experimental results.

Figure 4. (a MMC) O atoms adsorbed at different sites on the surface of 1 ¡Á 1 Ti3C2.Na atoms are adsorbed at the most stable positions on the (d) Ti3C2Tx and (e) Ti3C2Tx/Na3TCM surfaces of 3 ¡Á 3. (F) the diffusion curve of Na on the surface of Ti3C2Tx and Ti3C2Tx/Na3TCM in NEB calculation.
V. Assembly and Electrochemical performance of Ti3C2Tx/Na3TCM//AC Sodium Ion Capacitor
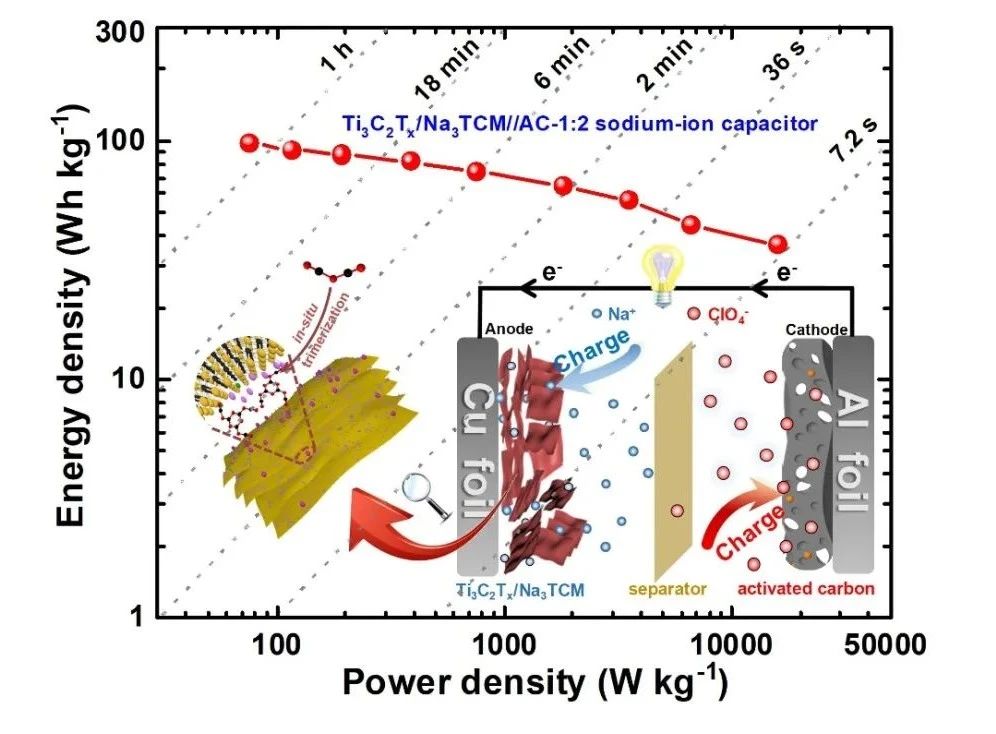
In order to better evaluate the electrochemical performance of Ti3C2Tx/Na3TCM, a sodium ion capacitor was assembled with Ti3C2Tx/Na3TCM as anode and commercial activated carbon (AC,YP80F) as cathode, and its capacitance characteristics were tested in a high voltage window of 04V. By optimizing different anode / cathode mass ratio, the sodium ion capacitor Ti3C2Tx/Na3TCM//AC-1:2 with the best performance was obtained. The calculation of the integral curve of constant current charge-discharge shows that the energy density of the sodium ion capacitor is 97.6 W/kg when the power density is 76 kW/kg, and 36.6 Wh/kg when the power density increases to 16.5 Wh/kg. It is worth noting that when Ti3C2Tx/Na3TCM//AC-1:2 completes the rapid charge-discharge process within 36 s, it can still reach a high energy density of 50 Wh/kg. In addition, Ti3C2Tx/Na3TCM//AC-1:2 showed excellent capacitance retention of about 90.8% after 4000 cycles and about 82.6% after 8000 cycles.

Figure 5. (a) charge storage mechanism of Ti3C2Tx/Na3TCM//AC NIC. (B) comparison of CV curves of Ti3C2Tx/Na3TCM//AC NIC with different anode / cathode mass ratios. (C) Ragone diagrams of Ti3C2Tx/Na3TCM//AC NIC and advanced LIC and NIC based on MXene electrodes. (d) capacitance retention and Coulomb efficiency of Ti3C2Tx/Na3TCM//AC NIC.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd ¾©ICP±¸16054715-2ºÅ |