
hotline£º
17715390137
Tel/Wechat£º
18101240246 (Technology)
0512-68565571
Email£ºmxenes@163.com £¨Sales Engineer£©bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

Research background.
Rechargeable secondary batteries have unique competitive advantages among the many alternatives to energy storage systems, such as higher theoretical capacity and energy density, long cycle life and higher working voltage. Assembling sodium-ion batteries and potassium-ion batteries with rich and low-cost sodium or potassium elements in the earths crust is the most potential ideal choice to replace the widely used lithium-ion batteries. However, due to the larger ion radius of sodium ion / potassium ion, the kinetic characteristics of sodium ion / potassium ion are slow in the process of electrochemical reaction, and serious volume changes will occur in the process of embedding / de-intercalation. Therefore, the performance of sodium ion battery and potassium ion battery can not reach the desired level. Therefore, there is an urgent need to explore high-performance electrode materials that can promote the movement and storage of larger alkali metal ions and improve battery performance, in order to meet the basic requirements of commercial applications.
Among the electrode materials of different dimensions, two-dimensional (2D) nanomaterials have unique advantages in rechargeable batteries, such as electrochemically active surfaces and interfaces, short ion diffusion paths and enhanced in-plane carrier / charge transport kinetics. Two-dimensional nanomaterials, such as transition metal oxides and transition metal chalcogenide compounds, have made some progress in the application of alkali metal ion batteries. However, some major problems still need to be solved, such as serious self-stacking, low conductivity, large irreversible capacity, low initial Coulomb efficiency and rapid capacity attenuation.
Recently, MXene, a new type of 2D nanomaterials derived from MAX phase materials, has been proved to be a potential electrode material in supercapacitors and rechargeable battery applications.Among them, Ti3C2Tx is a typical representative of MXene materials, and Tx represents the surface functional groups at the end, such as hydroxyl (- OH) and fluoro (- F) and so on. The advantages of ultra-high electronic and ion conductivity, controllable interlayer spacing and mechanical stability of MXene are needed for energy storage devices. However, MXene is faced with the problems of serious reaccumulation, large volume change, rapid capacity decay and relatively low capacity, which are common in 2D materials. Therefore, it is necessary to make full use of the advantages of physical and chemical properties of MXene materials to solve the above problems.
Brief introduction of achievements.
Recently, Professor Sun Zi Qi of Queensland University of Science and Technology in Australia, Dr. Li Junzhi of Nankai University, Wang Lili, a researcher at Institute of Semiconductors of the Chinese Academy of Sciences, and Professor Han Wei of Jilin University jointly published a research paper entitled Microbe-Assisted Assembly of Ti3C2Tx MXene on Fungi-Derived Nanoribbon Heterostructures for Ultrastable Sodium and PotassiumIon Storage in the internationally renowned academic journal ACS Nano, reporting a simple microbial-driven assembly strategy. A porous 2D MXene adsorbed on nitrogen-doped carbon nanofibers derived from 1D fungi was successfully prepared, which effectively alleviated the problems of self-stacking, volume expansion and rapid capacity decay of MXene materials in larger sodium and potassium ion battery applications.
Brief introduction of achievements.
Recently, Professor Sun Zi Qi of Queensland University of Science and Technology in Australia, Dr. Li Junzhi of Nankai University, Wang Lili, a researcher at Institute of Semiconductors of the Chinese Academy of Sciences, and Professor Han Wei of Jilin University jointly published a research paper entitled Microbe-Assisted Assembly of Ti3C2Tx MXene on Fungi-Derived Nanoribbon Heterostructures for Ultrastable Sodium and PotassiumIon Storage in the internationally renowned academic journal ACS Nano, reporting a simple microbial-driven assembly strategy. A porous 2D MXene adsorbed on nitrogen-doped carbon nanofibers derived from 1D fungi was successfully prepared, which effectively alleviated the problems of self-stacking, volume expansion and rapid capacity decay of MXene materials in larger sodium and potassium ion battery applications.
Guided reading of picture and text
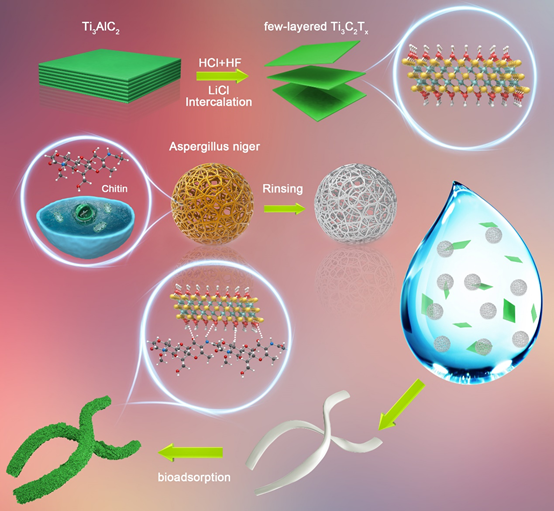

Figure 1.
The schematic diagram of the synthesis process of MXene@NCRib composite aerogels and the physical diagrams of different synthesis stages.

Figure 2.
The SEM images of the composites were as follows: (a) multilayer Ti3C2Tx MXene; (b) Aspergillus Niger; (c) MXene@NCRib composite aerogel.
Figure 2 shows scanning electron microscope images of Ti3C2TxMXene, Aspergillus Niger biofungal nanoribbons and 2D/1D MXene@NCRib composite aerogels. As shown in figure 2a, Ti3C2TxMXene etched with mixed acid has obvious layered structure. As an efficient intercalation agent, LiCl can be used to prepare colloidal dispersion of MXene with high concentration and excellent dispersion. Figure 2B shows the twisted shape and smooth surface of natural Aspergillus Niger nanoribbons, which are transformed into nitrogen self-doped carbon matrix after calcination at high temperature. This nano-banded fungus contains a large number of hydroxyl and amino groups at the end of chitin in the cell wall, which can ensure a high degree of chemical adhesion between it and the hydroxyl functional groups on the surface of Ti3C2Tx MXene. Therefore, MXene and fungal nanoribbons can be combined with each other through hydrogen bonds formed between MXene and biological macromolecules.After pyrolysis at high temperature, fungal nanoribbons were transformed into nitrogen-doped carbon NCRib. At the same time, MXene remained unchanged because of its inherent thermodynamic stability. As shown in figure 2C, Ti3C2Tx nanosheets are uniformly attached to the surface of NCRib to form a MXene@NCRib composite structure. It is worth mentioning that MXene nanowires are not stacked on the surface of the nanoribbons in a layer-by-layer assembly mode, but in a way of staggered assembly in the vertical direction, forming a self-supporting porous structure on the surface of fungal nanoribbons.This porous structure helps to take full advantage of the 2D structure of MXene. 2D/1D heterostructure combines the advantages of internal 1D conductive carbon nanoribbon network with the structural characteristics of external 2D nanowires, which not only has outstanding electronic conductivity, but also can promote the rapid diffusion of electrolyte ions.
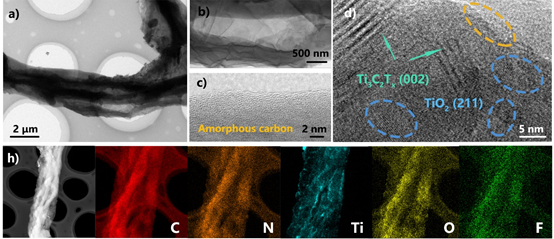
Figure 3.
Perspective TEM images and element distribution of MXene@NCRib composite aerogels: (a) magnified transmission image of MXene@NCRib; (b) MXene@NCRib; (c) transmission image of amorphous carbon; (d) high resolution transmission image of composite structure; (e) element distribution image of composite structure.
The microstructure of MXene@NCRib was further characterized by transmission electron microscope images. From figure 3, a core-shell composite structure of a folded Ti3C2Tx nanosheet coated on the surface of carbonaceous nanoribbons can be clearly observed. A more detailed surface structure can be observed in figure 3b, the mesoporous distribution formed by properly stacked flake Ti3C2Tx. This pore size distribution can provide sufficient electrochemical active sites, which can promote the transport kinetics of ions and electrons. With regard to the amorphous nature of bio-derived nitrogen-doped carbon nanoribbons, it can be proved from the disordered lattice stripes in figure 3C that nitrogen-doped amorphous carbon can play a positive role in catalysis and energy storage capacity in energy applications. As for Ti3C2Tx MXene nanowires, the characteristic {002} planes with lattice spacing of about 1.1nm were exposed in the high-resolution transmission HRTEM images (figure 3D). At the same time, the exposed TiO2 {211} crystal plane can be identified, which is caused by the slight oxidation of titanium atoms on the MXene surface during high temperature annealing. Figure 3e reflects the element mapping of the prepared 2D/1D heterostructure, including carbon, nitrogen, titanium, oxygen and fluorine. The uniform distribution of each element in the heterostructure proves the successful preparation of the composite.
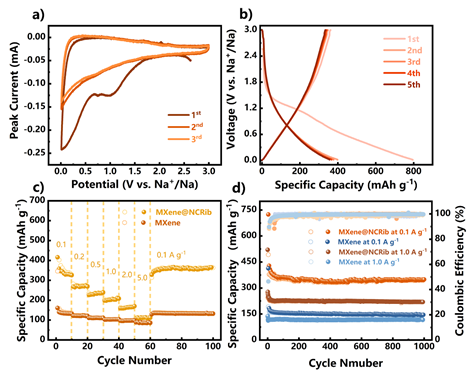
Figure 4.
The electrochemical performance of MXene@NCRib as negative electrode of sodium ion battery is as follows: (a) the first three CV cycles at the scanning rate of 0.2 mV Smurl; (b) the GCD curve at 0.1AgMel-1 current density; (c) the rate performance at different current densities; (d) the cycle stability at 0.1AgMuel-1 and 1.0 AgMuel-1 current densities.
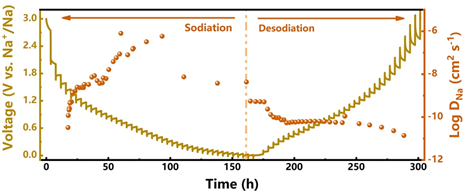
Figure 5.
GITT curve and sodium ion diffusion coefficient of MXene@NCRib heterostructure.
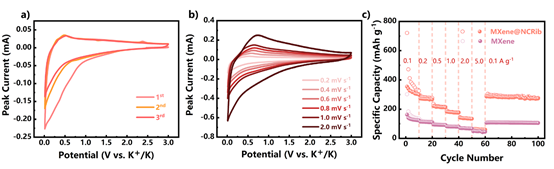
Figure 6.
The electrochemical performance of MXene@NCRib heterostructure as negative electrode of potassium ion battery: (a) the CV curve of the first three cycles at 0.2 mV Smurl scanning rate; (b) the CV curve at different scanning rate; (b) the rate performance under different current density.
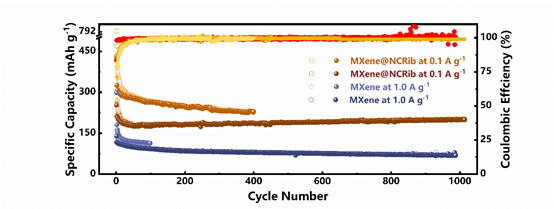
Figure 7.
The MXene@NCRib heterostructure is used as the cyclic stability of the negative electrode of potassium ion battery.
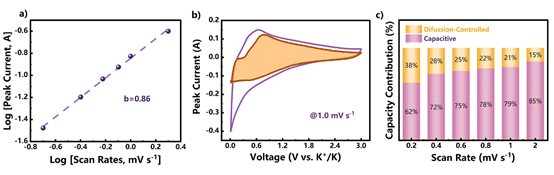
Figure 8.
Kinetic analysis of MXene@NCRib heterostructure potassium ion storage: (a) the logarithmic relationship between peak current and scanning rate; (b) the CV curve at 1.0 mV / smurl scanning rate, the shadow part is the proportion of capacitance contribution; (c) the proportion of different capacity contribution at different scanning rate.
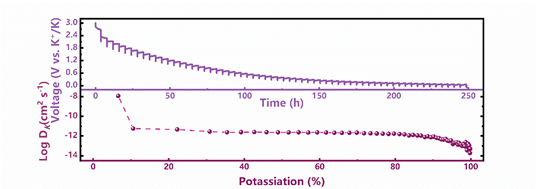
Figure 9.
GITT curve and potassium ion diffusion coefficient of MXene@NCRib heterostructure.
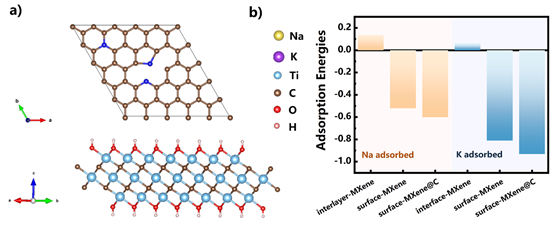
Figure 10.
(a) optimized crystal structure of nitrogen-doped carbon and MXene; (b) adsorption energy under different adsorption modes.
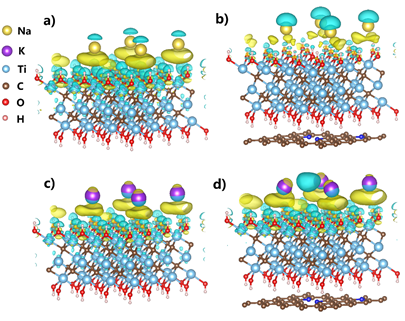
Figure 11.
Differential charge density of monolayer MXene and MXene@NCRib heterostructures in the process of sodium / potassium adsorption.
As shown in figure 11, in the results of differential charge density analysis of sodium / potassium adsorption at the interface and surface of Ti3C2 (OH) 2 and Ti3C2 (OH) 2@NCRib heterojunctions, the yellow and green electron clouds represent electron-rich and electron-deficient regions, respectively. These differences visually show the large amount of charge consumption around sodium / potassium ions adsorbed on the heterostructure surface. Thus it is proved that there is a large amount of charge transfer from sodium / potassium atom to 2D surface. Among them, the nitrogen-doped carbon matrix greatly promotes the charge transfer, which proves our previous hypothesis, that is, the synergistic effect of the designed heterostructure in the application of electrochemical energy storage.
This paper summarizes.
In this paper, a nitrogen-rich carbon nanofiber heterostructure derived from 2D/1D MXene@ fungi was synthesized by microbial-assisted assembly strategy using high chemical adhesion and nitrogen self-doped microbial fungi. Fungi are converted into nitrogen-doped carbon nanofibers, and 2D MXene is tightly assembled on the surface of nanofibers to form a self-supporting porous structure, so it can make full use of the structural advantages of 2D MXene and 1D conductive fibers. Thanks to the synergistic effect between the two, the porous 2D MXene structure and the rapid electron transfer in 1D nitrogen-doped carbon nanonetworks ensure the surface-controlled energy storage behavior and greatly enhance the electrochemical adsorption of sodium / potassium ions on the heterostructure surface. The 2D/1D MXene@NCRib electrode showed high reversible capacity and ultra-stable cycle performance: in the sodium / potassium ion battery, the high reversible capacity of 363.4 mAh / g Mel 1 and 287.2 mAh g Mel 1 at the current density of 0.1 A g muri 1, respectively, and the sodium / potassium storage capacity of 210.2 mAh g Muel 1 and 201.5 mAh g Muir 1 could be retained after 1000 high rate charge / discharge cycles. From the GITT test results, the excellent battery performance is mainly attributed to the ultra-fast ion transport and surface adsorption in 2D/1DMXene@NCRib heterostructure electrodes, which improves the slow electrochemical kinetic characteristics of large-size alkali metal ion rechargeable batteries. The microbial-assisted MXene@NCRib heterostructure assembly strategy used in this paper provides a new idea for the structure design of energy materials to solve the main challenges of 2D materials in energy storage applications.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd ¾©ICP±¸16054715-2ºÅ |