
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

管理员【微信QQ同步521006914】官方网站(www.TL9043.com)腾龙国际客服24小时在线,【公司直属客服】【公司直属开户】【大额无忧】支持视频验证现场。
Research Background
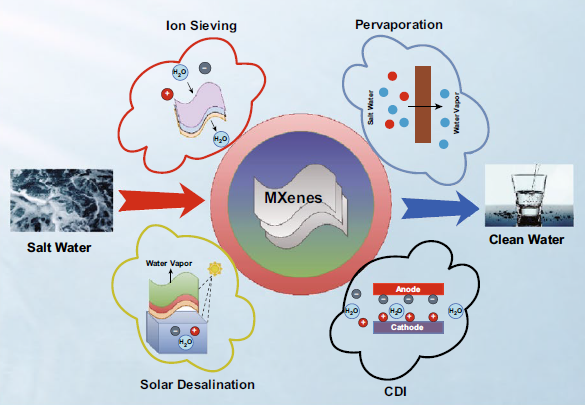
MXenes is a new 2D transition metal carbide, which also belongs to the family of nitrides and carbonitrides, which was discovered by researchers at Drexel University in 2011. A large number of recent studies have shown that MXene can be used for water purification, including capacitive deionization, membrane and adsorption. This review focuses on the synthesis and application of MXenes and MXene-based composite materials in seawater desalination and future challenges.
Potential of MXenes in Water Desalination: Current Status and Perspectives
Ihsanullah Ihsanullah
Nano-Micro Lett. (2020) 12:72
https://doi.org/10.1007/s40820-020-0411-9
Highlights of this article
1 The application of MXene and MXene-based nanomaterials in seawater desalination is reviewed.
2 Summarized the latest progress in the synthesis of MXene for seawater desalination. The desalination mechanism and cycling ability of MXenes are described.
3 Summarized the challenges and future prospects of MXene materials in seawater desalination. picture
brief introduction
MXenes are new 2D transition metal carbides with high surface area, high metal conductivity, easy functionalization and hydrophilicity, making them the best materials for energy storage applications. Recently, MXenes has also shown excellent application potential in water treatment and desalination. In this review, Ihsanullah Ihsanullah of King Fahd University of Petroleum and Minerals in Saudi Arabia summarized the latest progress in the synthesis of MXenes and MXene-based composites for seawater desalination. picture
Graphic guide
I. MXenes as ion sieve membrane
MXene nanosheets can be used for free-standing or supporting membrane size retention and charge selective retention of molecules and ions. The principle is that the interlayer spacing of MXene is limited, so smaller ions in the water can be eliminated. The water flux through the MXene (such as Ti3C2Tx) base membrane is very high, and based on the ion charge and hydration radius, the membrane shows excellent separation potential for various ions. There are studies on the preparation of Ti3C2Tx membranes by the vacuum assisted filtration (VAF) method, and a method for selective cation sieving has been reported. The thickness of the membrane plays a key role in the permeation of water through the membrane. The flux through the MXene membrane decreases as the thickness increases, until it reaches a steady-state value at a higher thickness. Ion permeability depends on the size and charge of the cation. MXene film has better selectivity to charged metal cations and dye cations of different sizes than graphene oxide.
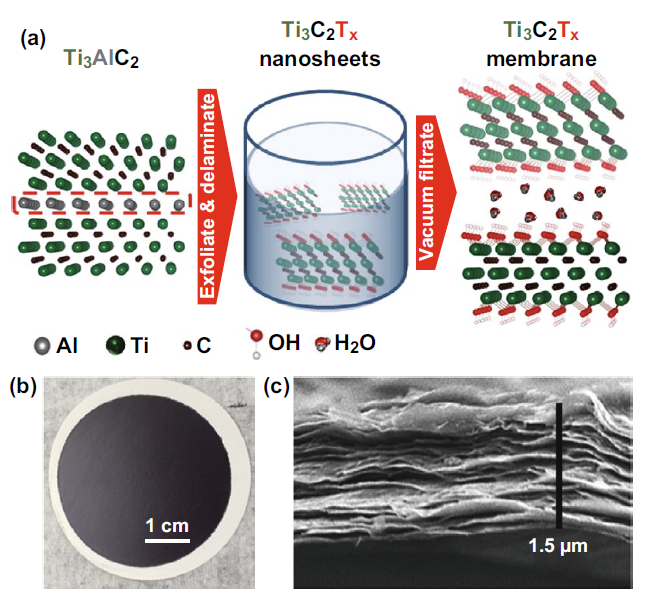
Figure 1. Synthesis of Ti3C2Tx film.
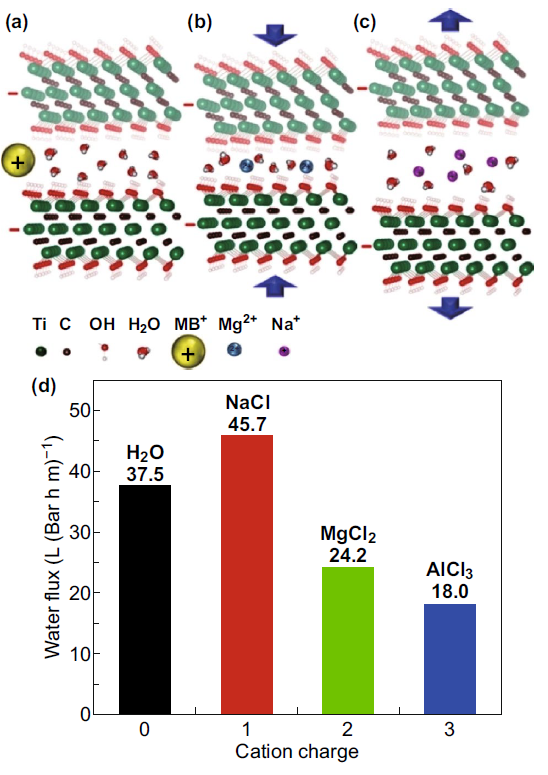
Figure 2. Separation ability of Ti3C2Tx membrane.
II. MXenes capacitive deionization (CDI)
CDI is an energy-saving electrochemical process in which anions and cations are separated based on their charges and deposited on a positively charged anode and a negatively charged cathode, respectively. The choice of electrode material is very important in the CDI process. The ideal electrode must have a higher surface area, good conductivity and higher stability. Due to its excellent capacitance characteristics, MXenes has become a new type of electrode material for CDI applications. CDI electrodes based on MXene have excellent cation and anion adsorption capacity due to their high conductivity, hydrophilicity and adjustable surface. Srimuk et al. introduced a CDI electrode based on MXene for the first time by casting Ti3C2 onto the porous separator of a CDI battery. The MXene CDI electrode showed excellent performance in 30 cycles, with a salt adsorption capacity of 13±2 mg/g (1.2 V battery voltage in a 5 mM NaCl salt solution). The adsorption of ions on the electrode occurs through ion insertion rather than double layer formation, as shown in Figure 3.
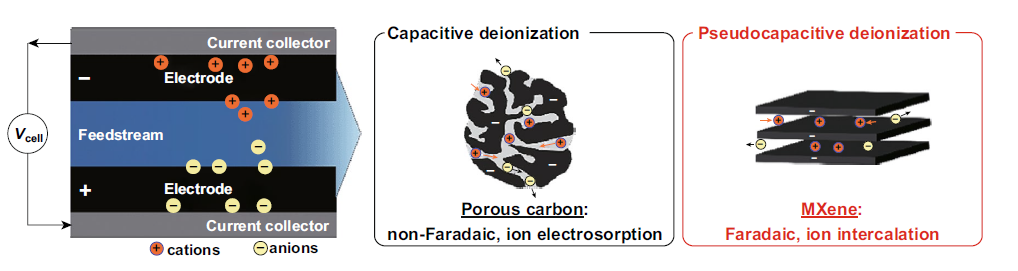
Figure 3. The concept of electrochemical water desalination by Faraday (electrostatic) ion adsorption on the surface of electrode materials.
III. MXenes used in seawater desalination
MXene has excellent light-to-heat conversion efficiency, making it an ideal choice for solar desalination applications. MXenes ability to evaporate light and hot water is another energy-saving feature of these 2D materials. Zhao et al. reported the synthesis of hydrophobic MXene film and the potential for solar desalination. As shown in Figure 4, layered Ti3C2 (d-Ti3C2) was obtained from the MAX phase by HCl/LiF etching, and then subjected to vacuum deoxidation and ultrasonic treatment. The hydrophobic film is obtained by surface modification of d-Ti3C2 with trimethoxy. The hydrophobic MXene film obtained after modification is used in solar evaporation equipment, which self-floats on seawater. The membrane achieves a solar vapor conversion efficiency of 71%, a solar evaporation rate of 1.31 kg·m2/h, and stability within 200 h under high salinity conditions. The rejection rate of the four main ions (Ca2+, Mg2+, Mg2+ and Na+) exceeds 99.5%, while for organic dyes and heavy metals, it reaches a rejection rate of nearly 100%, as shown in Figure 5. Due to poor salt blocking after long-term use, it is not suitable for long-term solar desalination applications.
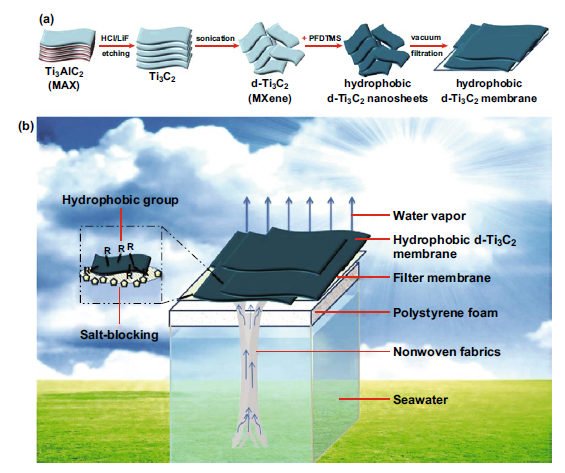
Figure 4. Schematic diagram of the manufacturing process of the hydrophobic d-Ti3C2Tx film and the solar desalination device.
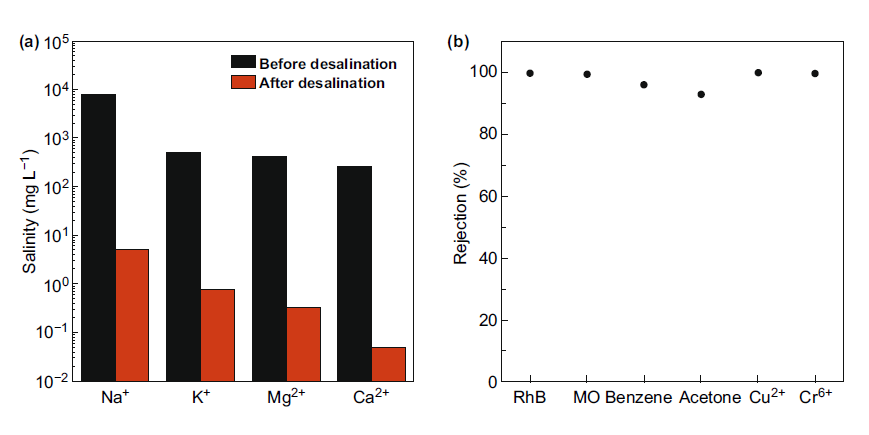
Figure 5. (a) The salinity of the four primary ions before and after solar desalination. (b) Organic and heavy metal ion repellency.
IV Total evaporation desalination
Pervaporation desalination is a combination of water that diffuses through the membrane and then evaporates into the gas phase on the other side of the membrane. The MXene/PAN composite material and the free-standing MXene membrane are made by vacuum filtration of the MXene suspension, as shown in Figure 6. A certain amount of MXene nanosheets are deposited on the PAN substrate to prepare MXene/PAN composite materials, while a similar method is used to prepare a separate MXene film using polycarbonate (PC), and then the MXene is peeled off on the dried substrate . As the number of MXene nanosheets increases, the thickness of the MXene layer also increases, and its color changes from light green to dark green. These membranes have great potential for pervaporative desalination, which can remove 99.5% of salt at a temperature of 95% and show a water flux of 85.4 L/(m2·h). Compared with other membranes reported in the literature, MXene membrane also shows excellent desalination performance for seawater. One of the disadvantages of pervaporation desalination is the large-scale use of energy. However, with the use of renewable energy or waste-source energy, energy efficiency is expected to be significantly improved.

Figure 6. MXene film for pervaporation desalination.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |