one. Article overview
Structural two-dimensional transition metal carbides and/or nitrides (MXenes) have attracted the attention of the material science research community due to their unique physical and chemical properties. However, a simple and cost-effective synthesis of MXene has not yet been reported. Here, using elemental precursors, we report a method of forming aluminum carbide from titanium carbide and subsequently synthesizing MXene by in-situ etching in a molten salt tank. Molten salt is used as the reaction medium to prevent the oxidation of the reactants during the high-temperature synthesis process, so that MXene can be synthesized in an air environment that does not use inert gas protection. The Ti3C2Tx terminal Cl and Ti2CTx MXene are prepared by a one-pot synthesis method, where the 700°C in-situ etching step only takes about 10 minutes. In addition, when used as an active material for non-water lithium ion storage in a half-cell structure, the obtained Ti2CTx MXene exhibits lithiation capacities of approximately 280mAhg-1 and 160mAhg-1 at specific currents of 0.1Ag-1 and 2Ag-1, respectively value.
Two, graphic guide
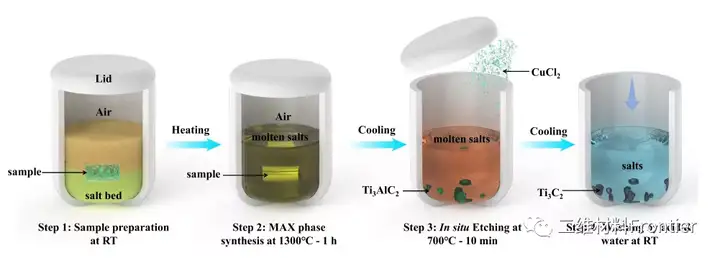
Figure 1. One-pot synthesis of two-dimensional titanium carbide in air.
Schematic diagram of single-pot synthesis of Ti3C2Tx Mxene in the open air using Ti, Al, and C element powders as raw materials. RT stands for room temperature. The sample particles contained Ti:Al:C:NaCl:KCl powder with a molar ratio of 3:1.2:1.9:3:3; the salt bed was composed of a mixture of sodium chloride and potassium chloride with a molar ratio of 1:1. The synthesis time of the whole process is 460 minutes.
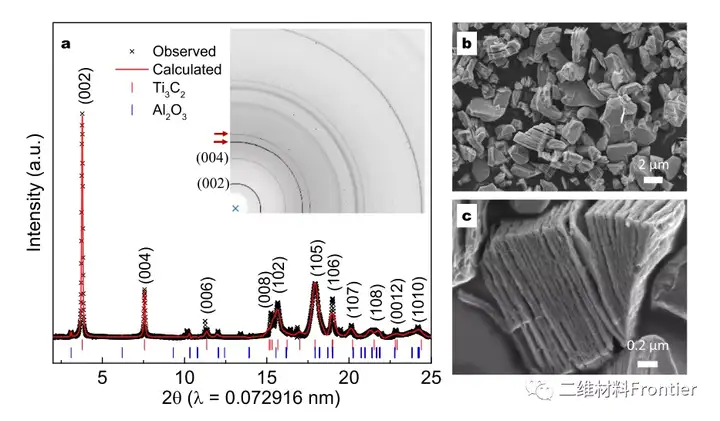
Figure 2. Structural characterization of Ti3c2Tx.
a is 1D and 2D synchrotron ray (λ=0.072916nm) diffraction (SXRD) mode and Rietveld refinement of Ti3C2Tx prepared by etching at 700°C for 10 min). The red arrow in the two-dimensional pattern points to the diffraction peak of the sample holder; b and c are SEM images of Ti3C2Tx, and the scale bars are b-2μm and c-0.2μm, respectively.
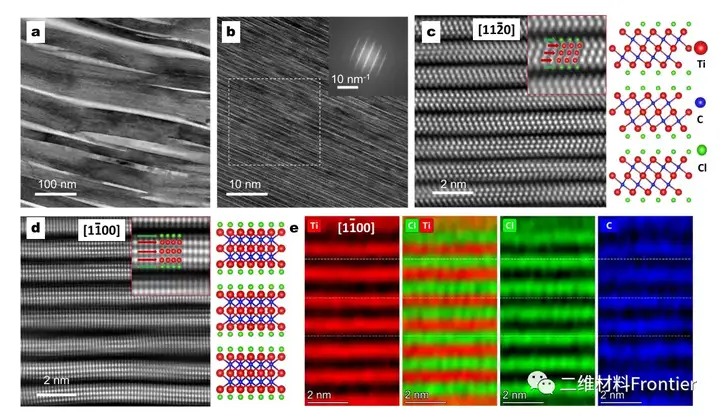
Figure 3. Atomic structure analysis of Ti3C2Tx MXene prepared by etching at 700°C for 10 minutes.
A and b high-resolution transmission electron microscope images, where the inset shows the fast Fourier transform (FFT) mode of the selected area, and the scale bar is 1/10nm. Atomic resolution high-angle annular dark field (HAADF) image with c[1120] and d[1100] projections and the corresponding crystal structure; the inset is a magnified view of the position of the atom. e EDS mapping of atomic scale EDS along the projection of [1100].
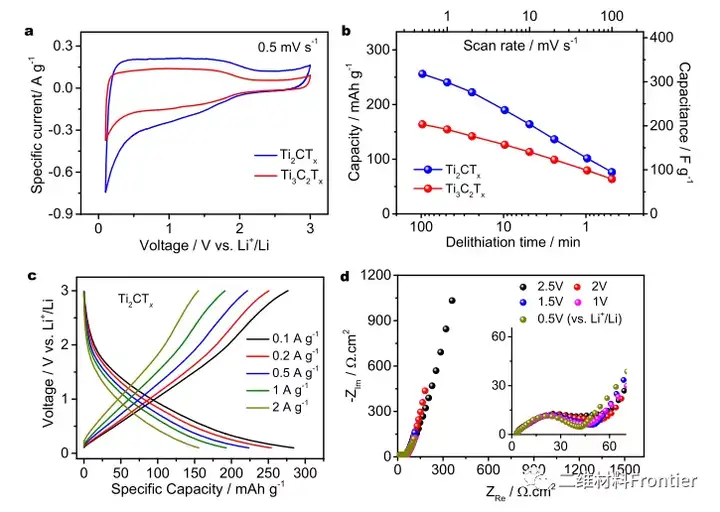
Figure 4. Electrochemical energy storage performance of Ti3C2Tx- and T2ctx-based electrodes.
a is the cyclic voltammetry spectrum of Ti3C2Tx and T2CTx MXene at 0.5mV/s; b is the specific capacity comparison of Ti3C2Tx and T2CTxMXene at different scan rates; c is the voltage spectrum of Ti2CTx MXEne electrodes at different specific currents; d and Ti2CTx at different potentials Electrochemical impedance measurement of MXEne electrode.
3. Full text summary
This article proposes a simple one-pot synthesis of element precursors to prepare MXene. By eliminating the need for inert gas protection, the synthesis operation greatly simplifies the synthesis operation. Compared with the traditional MXene synthesis method prepared separately, the single-pot synthesis method shortens the entire synthesis time. The cl end Ti3C2Tx and T2CTx MXenes use a fast etching step at 700°C, only 10 minutes, and the overall synthesis time is less than 8h. Lithium ion storage studies have shown that the one-pot synthesis of MXenes and the previously reported MXenes obtained by Lewis molten acid etching have similar electrochemical characteristics. The obtained Ti2CTx MXene provides lithiation capacity values of approximately 280mAhg-1 and 160mAhg-1 at specific currents of 0.1Ag-1 and 2Ag-1, respectively. We believe that the single-pot synthesis method paves the way for the rapid reduction of production costs of MXene materials, revealing the broad potential of MXene materials in energy storage applications.
Article link:
https://doi.org/10.1038/s41467-021-25306-y
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.