
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online



one. Background introduction
Approximately 10 million cancer patients die each year, accounting for more than 15% of global deaths. 1 Therefore, there is an urgent need to find new anti-cancer treatments. Current anti-cancer treatments often have undesirable side effects, and natural anti-cancer drugs have been proven to be less effective, requiring higher local concentrations to induce anti-cancer properties. Lactoferrin has a variety of therapeutic properties, including anticancer, antibacterial, antiparasitic, antiviral, and antifungal activities. 2 It has recently been discovered that lactoferrin can increase the recovery of patients infected with COVID-19. 4 This transferrin protein exhibits these therapeutic activities by recruiting immune-related cytokines and isolating and consuming nutrients in the diseased area through metal ions. Protease digested lactoferrin usually results in fragments that exhibit improved therapeutic activity. Compared with full-length lactoferrin (flHLF), lactoferrin (peptide digested lactoferrin) has better antibacterial and antifungal properties.
Two, graphic guide
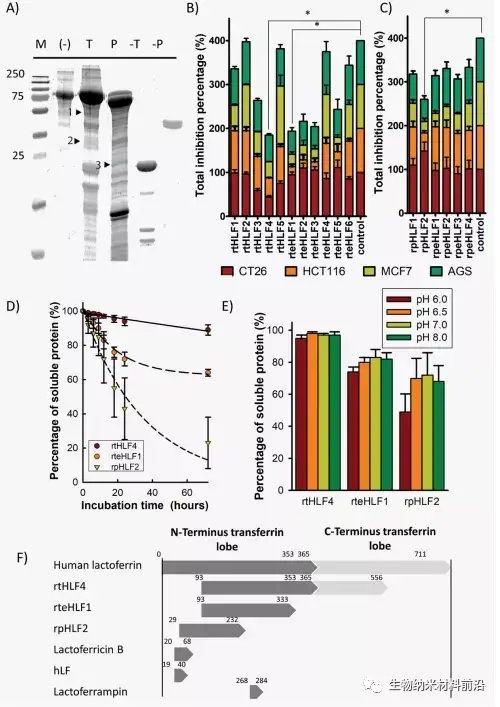
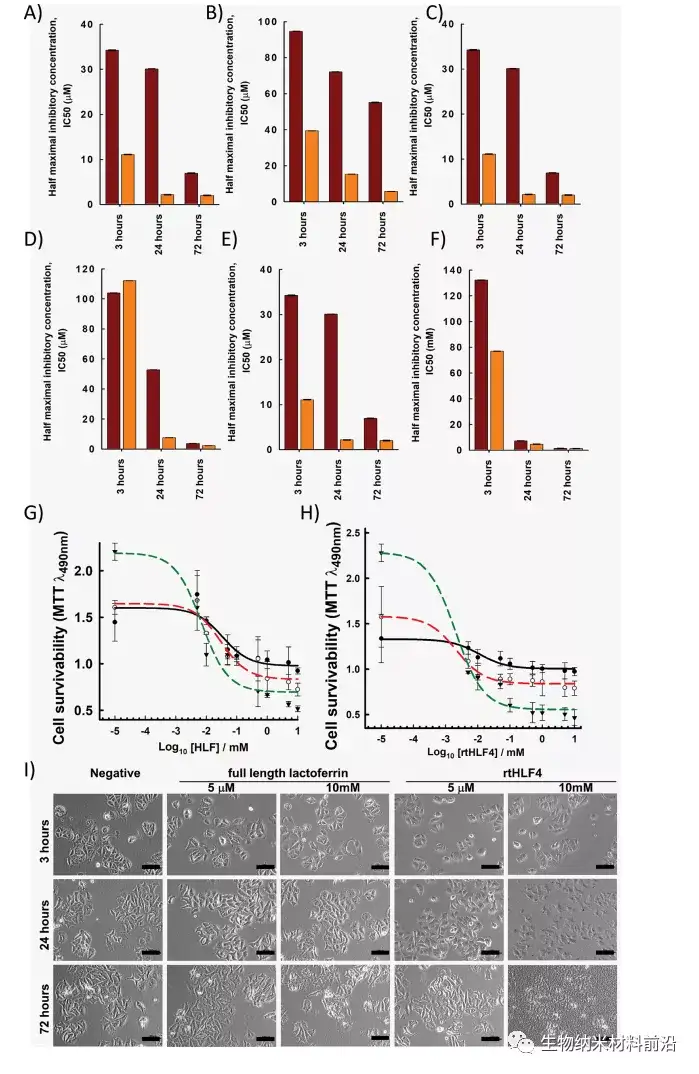
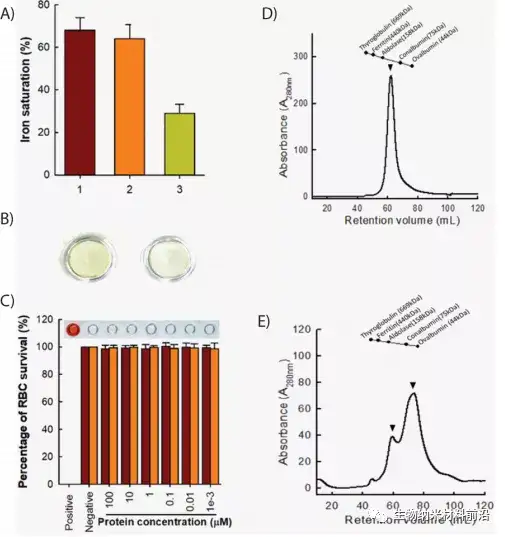
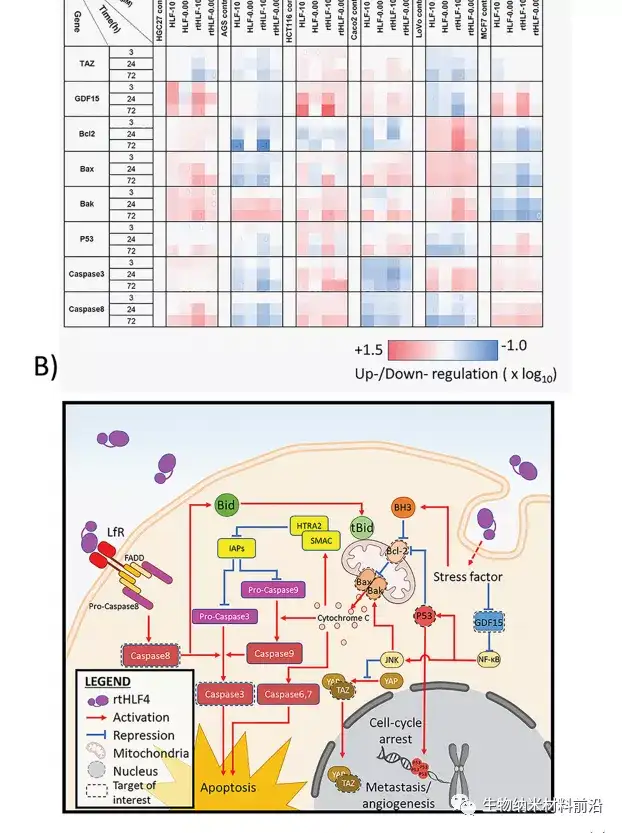
Figure 3. Anti-cancer mechanism of gastric cancer, colon cancer and breast cancer cells after flHLF and rtHLF4 treatment. (A) Quantitative PCR results of different genes in gastric cancer, colon cancer and breast cancer cells. The expression level was compared with the housekeeping gene GADPH (flHLF/rtHLF4-10: 10μM flHLF/rtHLF4; flHLF/rtHLF4 flHLF/rtHLF4-0.005: 0.005μM). (B) Different anti-cancer pathways of cancer cells triggered by rtHLF4 treatment.
3. Full text summary
Three protease digested human lactoferrin fragments were identified, which have better anti-cancer properties than flHLF. Among them, rtHLF4 has the best stability and tolerance to human physiological conditions. In further examination of the mechanism of rtHLF4, we found that rtHLF4 induces various cancer cell receptor interactions through lactoferrin types. rtHLF4 internalizes rtHLF4 and inhibits metastatic genes, thus indicating the potential use of this protein in future cancer treatments. Before rtHLF4 can be developed as a viable treatment, further research is needed to better understand its anti-cancer mechanism. These studies include more in-depth studies on receptor proteins and binding proteins involved in the internalization of rtHLF4 into the cytoplasm. We intend to further study this interaction, using thermal proximity co-aggregation to describe the complex dynamics of proteins in these cancer cells. 24rtHLF4 may also serve as the export mechanism of synthetic biology-driven cell engineering for anti-cancer therapy.
Article link:
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |