1. Article overview
This article proposes a simple and universal method for anchoring vacancies in monoatomic metal synthesis, which has good activation properties for peroxymonosulfate (PMS). The molten transition metal salt is used to generate Ti vacancy defects on the environmentally friendly Ti2AlN metal liner in one step, metal ions are converted into metal atoms, monoatomic metal anchors and two-dimensional (2D) MXene structures are constructed. In the PMS/monoatomic Co process, carbamazepine (CBZ), sulfamethoxazole (SMX) and 2,4-2 chlorophenol (2,4-DCP) are completely removed in less than 2 minutes. Type pollutants. Hydroxyl radicals (OH), sulfate radicals (SO4-) and non-radical oxidation together promote the degradation of pollutants. More importantly, the strong reducing Ti atoms of the bare MXene substrate trigger the Co(III)/Co(II) cycle, thereby accelerating the activation of PMS. The single-atom Co catalyst can show excellent performance even under alkaline conditions or in actual river water.
Two, graphic guide
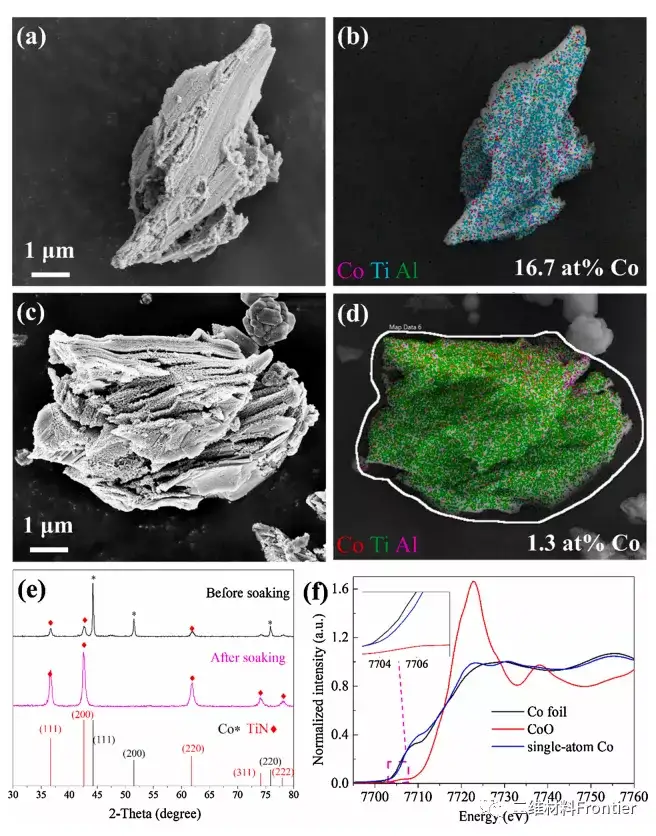
Figure 1. SEM images and EDS mapping images of monoatomic Co catalysts (a, b) and (c, d) immersed in 3M hydrochloric acid solution. (e) XRD patterns of monoatomic Co catalyst before and after immersion in 3M hydrochloric acid solution. (f) Normalized XANES of single-atom Co catalyst, Co foil and cobalt oxide at the Co k-edge.
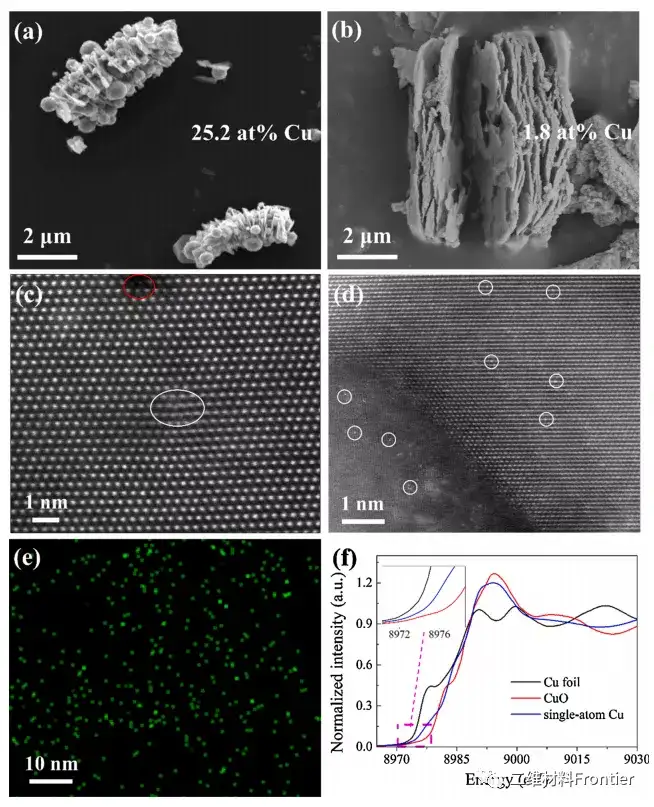
Figure 2. Scanning electron microscope images of monoatomic Cu catalysts (a) and (b) after immersing in 3M hydrochloric acid solution containing 50g/L ferric chloride. (c-d) ac-STEM-HAADF image of the monoatomic copper catalyst. (e) EDS mapping image of monoatomic Cu catalyst. (f) Normalized XANES of the copper k edge of the monoatomic copper catalyst, copper foil and copper oxide.
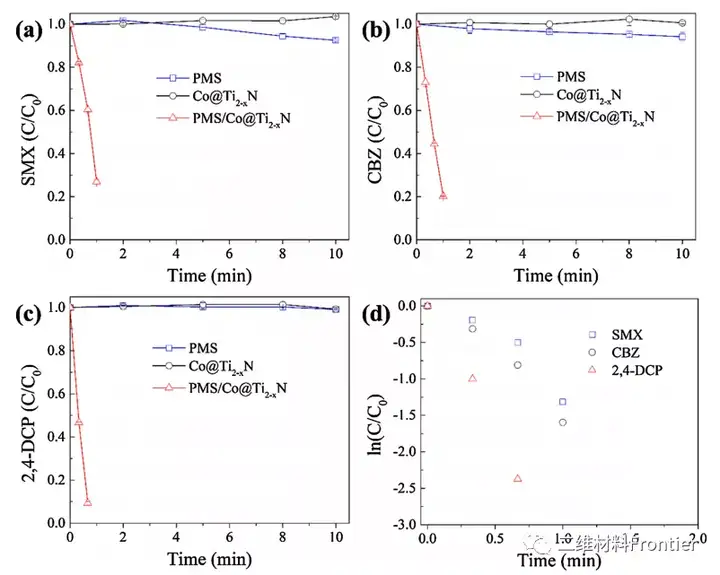
Figure 3. Degradation of (a) SMX, (b) CBZ and (c) 2, 4-DCP using the prepared Co@Ti2-xN catalyst. (d) The change of ln (C/C0) and time. Conditions: [Catalyst]0=50mgL-1, [PMS]0=2mM, [SMX]0=5μM, [CBZ]0=5μM, [2,4-DCP]0=5μM, [pH]0=6.95, T=25°C.
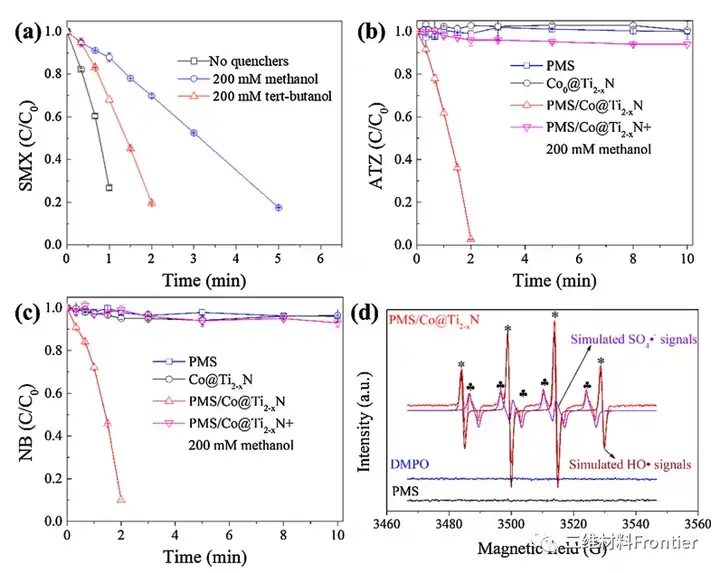
Figure 4. (a) The effect of methanol and tert-butanol on the degradation of SMX. (b, c) Degradation of ATZ and NB in the presence and absence of methanol. (d) EPR spectrum of Co@Ti2-xN catalyst, "*" represents the DMPO-HO addition signal (aN=15.02G, aβ-H=14.93G), ♣ represents the DMPO-SO4 addition signal (♣=13.80G) , Aβ-H=10.06G, aγ-H1=1.55G, aγ-H1=0.80G). Conditions: [Catalyst]0=50mgL-1, [PMS]0=2mM, [SMX]0=[ATZ]0=[NB]0=5μM, [pH]0=6.95 and T=25°C.
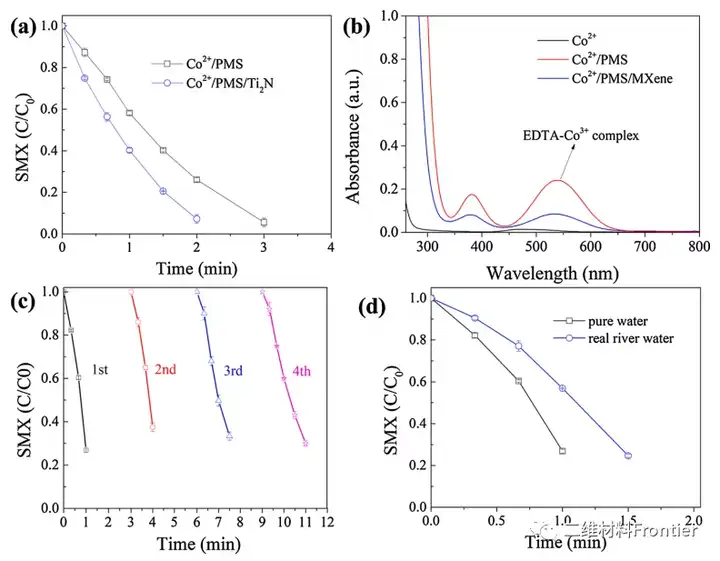
Figure 5. (a) Degradation of SMX in the Co2+/PMS process in the presence and absence of Ti2N MXene. (b) The UV-Vis spectrum of the Co2+/PMS process in the presence and absence of MXene. (c) Cyclic operation of Co@Ti2-xN catalyst. (d) Degradation of SMX in actual river water. Conditions: [Catalyst]0=50mgL-1, [CoCl2]0=5mgL-1, [Ti2N]0=10mgL-1[PMS]0=2mM, [SMX]0=5μM and T=25°C.
3. Full text summary
This article proposes a simple and universal method for the synthesis of monoatomic molten metal salts. This method uses a titanium vacancy defect anchoring strategy instead of a complex c-n-metal coordination method. Effective activation of the prepared monoatomic metal and ultra-rapid degradation of refractory organic pollutants. In addition to the two currently recognized PMS activation pathways, namely single-atom metal activation and non-radical oxidation, triggering the Mo(III)/Co(II) cycle to accelerate the activation of PMS is considered to be another unique approach. In addition, the degradation efficiency of single-atom Co activated PMS on organic pollutants is higher than that under acidic conditions, and is better than traditional Fenton-like reactions based on metal ions. After long-term recycling, the monoatomic cobalt catalyst still shows satisfactory performance. In actual river water, the monoatomic cobalt catalyst also maintains a good efficiency. MAX ceramics includes a family of materials, as many as hundreds of species. All transition metal cations with metal activity lower than the M and A atoms in MAX ceramics can be used to prepare monoatomic metals by the molten metal salt method. Therefore, this research not only develops the oxidation process of heterogeneous mass spectrometry, but also provides a general method for the convenience of synthesizing single-atom metal catalysts.
Article link:
https://doi.org/10.1016/j.apcatb.2021.119898
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.