已传文件:photo/1631586161.png
Fucoidan‑Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of CancerMi Hyeon Cho, Yan Li, Pui‑Chi Lo, Hyeri Lee, Yongdoo Choi*Nano-Micro Lett.(2020)12:47https://doi.org/10.1007/ s40820-020-0384-8
Highlights of this article
1 A therapeutic nanogel based on fucoidan, consisting of a fucoidan backbone, redox-responsive linkages and photosensitizers, which can activate fluorescence imaging of tumor sites, enhance the efficacy of photodynamic therapy, and ultimately induce The cancer died completely. 2Using fucose on the nanogel platform as a polymeric backbone can achieve tumor targeting through P-selectin binding, and enhance the anti-tumor effect by inhibiting the binding of vascular endothelial growth factor.
brief introduction
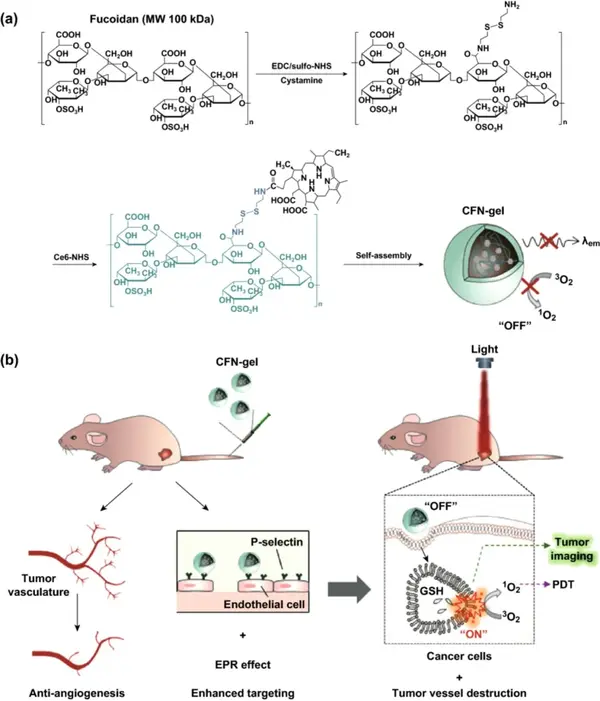
In this paper, a fucoidan-based therapeutic nanogel (CFN-gel) is developed. The gel is composed of a fucoidan backbone with a certain anti-tumor effect, a redox-responsive connection bond, and a photosensitizer Ce6. In order to achieve activatable near-infrared (NIR) fluorescence imaging and enhanced photodynamic therapy (PDT) of the tumor site, it can induce the complete death of cancer cells. CFN-gel has anti-angiogenesis and promotes tumor accumulation through P-selectin targeting and EPR effect. In addition, CFN-gel has no fluorescence and phototoxicity when administered systemically. After the cells are internalized, the optical activity of CFN-gel recovers with the change of the intracellular redox potential, thereby achieving selective near-infrared fluorescence imaging of tumors and enhanced PDT therapy. This shows that fucoidan nanogel is an efficient and specific new material for tumor imaging and treatment.
Graphic guide
Characterization of I CFN‑Gel
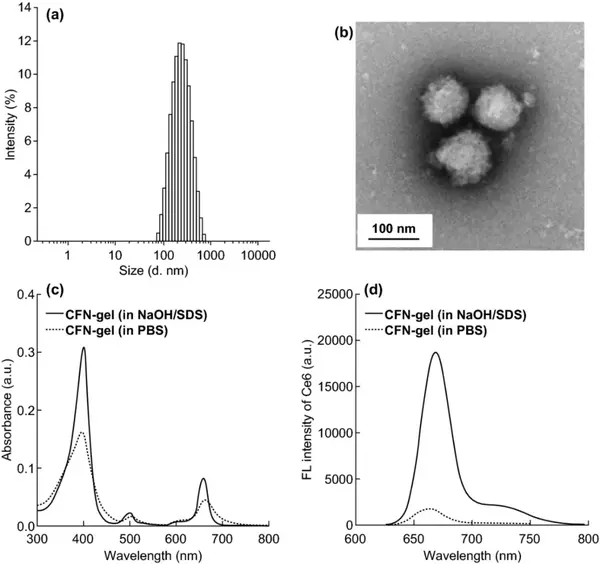
First, cystamine hydrochloride was used to modify the carboxylic acid of the fucoidan backbone; then Ce6 was combined into the amino-terminal fucoidan to prepare CFN-gel. According to 1H-NMR calculation, each fucoidan is bound with 20.3 Ce6 photosensitizer molecules. The Ce6-fucoidan conjugate can be self-assembled into nanogels after freeze-drying and redispersing in aqueous solution. Its hydrated particle size and zeta potential are 259±59 nm and -22.2 mV, respectively. The TEM image confirmed the formation of nanogels. CFN-gel has obvious aggregation leading to fluorescence quenching phenomenon. Compared with the CFN-gel in the surfactant solution, the fluorescence intensity of the CFN-gel in the PBS solution was quenched 10 times.
Figure 1 (a) Synthesis steps of Ce6-fucose nanogel (CFN-gel); (b) Schematic diagram of CFN-gel and its mode of action.
Figure 2 CFN-gel (a) distribution of hydrated particle size; (b) TEM image. Ultraviolet/visible absorption spectrum (c) of CFN-gel in a solution containing surfactant and PBS at 2 μM Ce6 equivalent (c); fluorescence emission spectrum (d).
II NIR fluorescence and SOG redox response
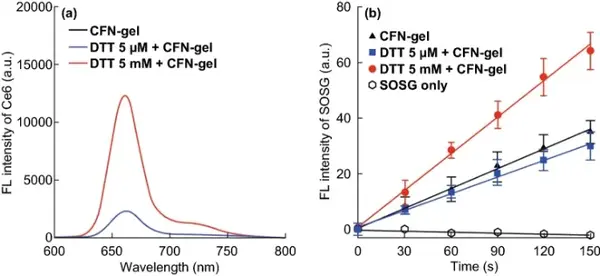
In the cell, the disulfide bonds in CFN-gel are cleaved by redox reactions to release Ce6 molecules, thereby inducing fluorescence emission and the production of SOG. When CFN-gel is treated with 5 mM DTT, its fluorescence intensity is 4.9 times higher than that of the gel without DTT or low-concentration DTT, indicating that after the disulfide bond is broken, the aggregated Ce6 molecules in the CFN-gel can be effectively released , Thereby turning on the fluorescence of CFN-gel. The singlet oxygen sensor green (SOSG) was used as the singlet oxygen detection reagent to analyze the SOG of CFN-gel under 670 nm light. After treatment with 5 mM DTT, the production of CFN-gel SOG was 1.9 times higher than that of CFN-gel treated with 0 or 5 μM DTT. These data indicate that CFN-gel has redox response fluorescence emission and phototoxicity.
Figure 3 The redox response of the fluorescence signal is turned on and the singlet oxygen generation of CFN-gel. (a) The fluorescence emission spectrum of CFN-gel treated with DTT for 4 h; (b) The fluorescence of SOSG of CFN-gel treated with DTT under 670 nm laser irradiation increased with time (n=4).
III SPR analysis of CFN-gel
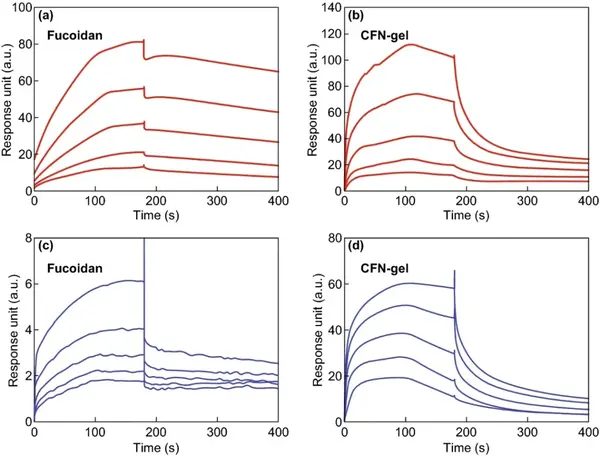
SPR analysis was used to compare the binding properties of CFN-gel with VEGF-165 and P-selectin. The equilibrium dissociation rate constants (KD) of VEGF-165 with fucoidan and CFN-gel are 953.0 and 756.1 nM, respectively. The KD values of P-selectin, fucoidan and CFN-gel were 69.71 and 718.9 nM, respectively.
Figure 4 Surface Plasmon Resonance (SPR) analysis.
IV in vitro cell research
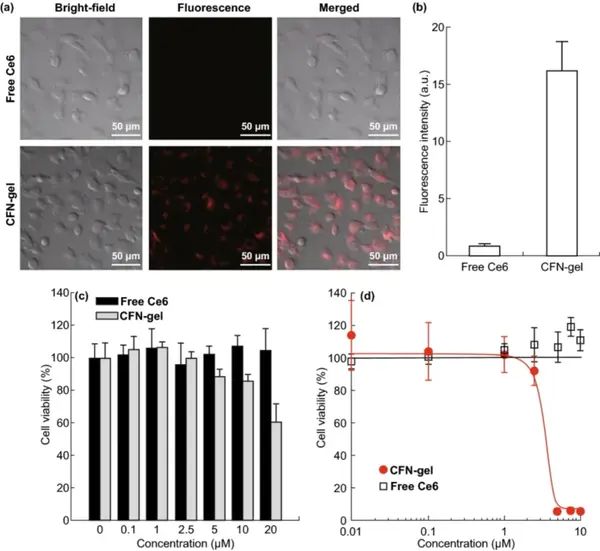
CFN-gel has good dispersion stability in cell culture media containing serum proteins. HT1080 tumor cell endocytosis experiments show that CFN-gel is effectively internalized into tumor cells. After the fluorescence-quenched Ce6 is released in the cell through a redox response, the fluorescence is activated. The cell uptake of CFN-gel is at least 18 times that of free Ce6. The CCK-8 method was used to compare the cell survival rate, and to explore the in vitro therapeutic effect of CFN-gel on HT1080 cancer cells. In the absence of light, at the test concentration, cancer cells treated with free Ce6 for 6 hours have no cytotoxicity. Similarly, at a concentration of 0.1 to 2.5 μM, it was observed that CFN-gel treated cancer cells were not cytotoxic. As the concentration of CFN-gel increased from 5 μM, 10 μM, and 20 μM, the cell viability gradually decreased to 88.3%, 85.9%, and 60.2%, respectively. CFN-gel treatment for 24 hours further reduced cell viability, while free Ce6 treatment at a concentration of up to 10 μM for 24 hours did not show any cytotoxic effects on tumor cells. Incubate HT1080 cells with CFN-gel or free Ce6 at different concentrations for 6 hours, irradiate with 670 nm, 10 J/cm2 laser (dose rate 50 mW/cm2), and continue to incubate for 24 hours. The IC50 of CFN-gel combined with Ce6 The value is approximately 2.73 μM. In contrast, free Ce6 treated cells have no therapeutic effect under light.
Figure 5 Free Ce6 and CFN-gel treatment of near-infrared fluorescence image of HT1080 cancer cells (a); quantitative analysis of fluorescence intensity (b); in vitro dark toxicity of free Ce6 and CFN-gel on HT1080 cells in vitro (c); In vitro phototoxicity under 670 nm light (50 mW/cm2, 10 J/cm2) (d).
V Near-infrared fluorescence imaging in vivo
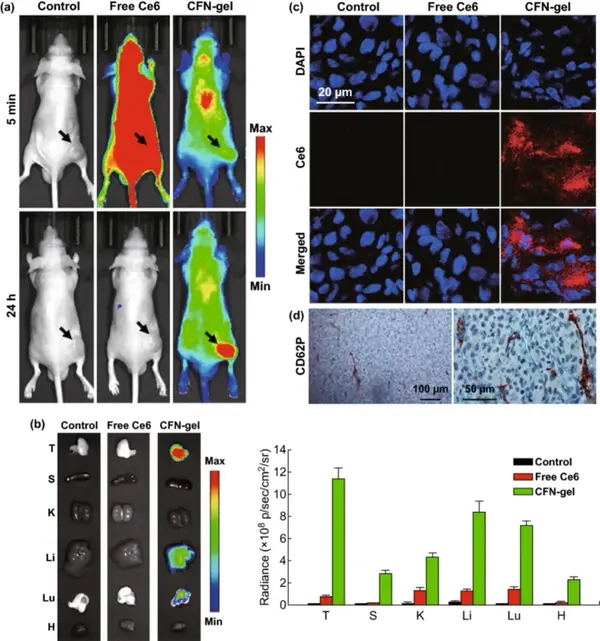
A xenograft mouse tumor model was used to evaluate the application of CFN-gel in tumor imaging in vivo. Near-infrared fluorescence images (λex=660/20 nm, λem=710/40 nm) were obtained 5 minutes and 24 hours after intravenous injection. Five minutes after injection, mice treated with free Ce6 showed high fluorescence intensity, while mice treated with CFN-gel showed low fluorescence signal. 24 hours after the injection, a small amount of fluorescence signal was observed in the mice treated with free Ce6, indicating that most of the Ce6 was cleared from the body, and there was no significant accumulation of free Ce6 in the tumor tissue. On the contrary, 24 hours after the injection, the CFN-gel treated mice could still observe the fluorescence signal all over the body, and the tumor site was significantly different from the surrounding normal tissues. It shows that the injected CFN-gel not only prolongs the blood circulation and tumor accumulation in tumor tissues, but also activates fluorescence, which is consistent with the results of in vitro studies. Collecting the main organs of the mice, it can be found that the mice treated with CFN-gel have strong fluorescence in the tumor site. According to CD62P staining of HT1080 tumor sections, high P-selectin expression was also observed, indicating that in addition to the EPR effect, the high expression of P-selectin in tumor tissues enhanced the tumor targeting of CFN-gel.
Figure 6 In vivo evaluation of selective tumor imaging in a xenograft tumor model.
VI CFN‑Gel s anti-tumor effect
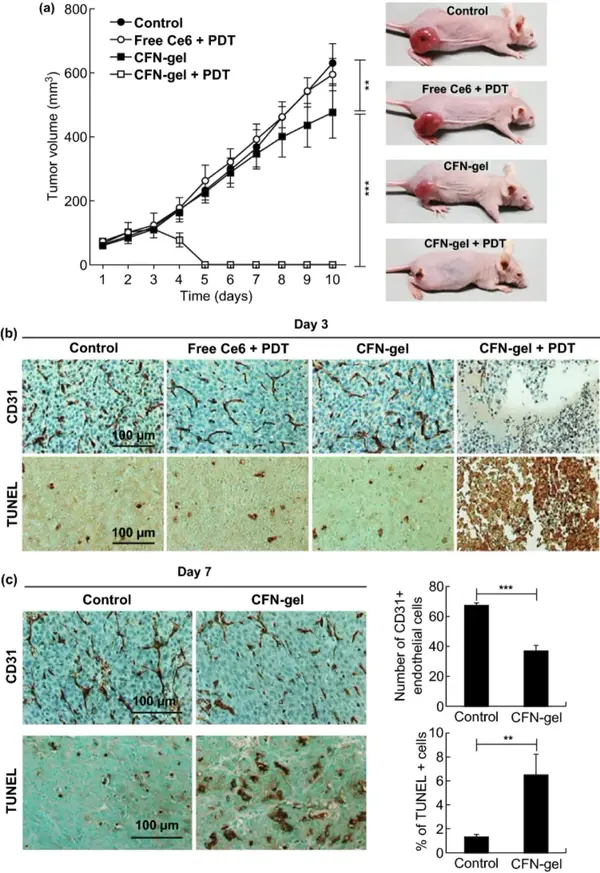
By measuring the tumor growth rate of the HT1080 xenograft tumor model, the anti-tumor effect of CFN-gel combined with PDT was further evaluated. Compared with the PBS group, the free Ce6+PDT group and the CFN-gel group, the CFN-gel+PDT group has a significantly enhanced treatment effect in vivo. The TUNEL experiment confirmed the significant therapeutic effect of CFN-gel and light. It may be that CFN-gel was internalized into the tumor vascular endothelial cells overexpressing P-selectin and destroyed them under light, resulting in CD31 staining of the fourth group of tumor sections, almost invisible angiogenic tumor blood vessels.
Compared with the PBS control group, mice using CFN gel alone showed delayed tumor growth at 7-10 days, which may be due to the anti-cancer potential of fucoidan itself. Quantitative analysis of immunohistochemical staining images showed that compared with the PBS group, the number of CD31-positive endothelial cells in the CFN-gel treatment group was reduced by 45.3% (***P<0.001), indicating that CFN-gel has anti-vascular properties Generate effect. Compared with the PBS group, the apoptotic cells in the tumors of the CFN-gel treatment group increased by 4.9 times (**P<0.01), which indicates that the accumulation of CFN-gel in the tumors induced anti-tumor effects.
Figure 7 Study on the anti-tumor activity of CFN-gel combined with PDT in vivo.
VII In vivo biocompatibility of CFN-gel
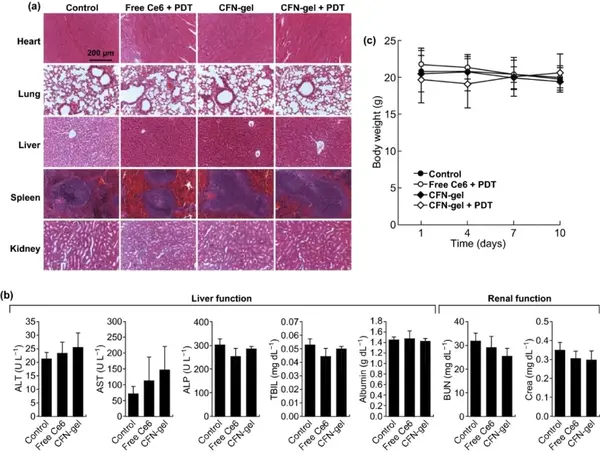
H&E staining of the tissue sections showed no histopathological abnormalities in the main organs, and the weight of the mice was not significantly reduced, indicating that CFN-gel has good biocompatibility.
Figure 8 In vivo biocompatibility of CFN-gel. (a) Representative H&E stained images of major organs collected from different treatment groups on day 10; (b) Blood chemistry data of mice in PBS, free Ce6 and CFN-gel treatment groups on day 10 (n=4); c) Changes in the average body weight of mice in different groups over time. In any treatment group, no obvious signs of organ, blood biomarkers, and body weight toxicity were observed.
Written by: Nawei Express Editorial Department
Editor: Nawei Express Editorial Department
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.