已传文件:photo/1631586161.png
Article information
Revealing the formation and energy storage mechanism of single crystal perovskite sodium niobate/small-layer niobium carbide MXene composite electrode for lithium ion capacitors First author: Qin Li, Liu Yang Corresponding author: Yuan Changzhou*, Hou Linrui* Unit: Jinan University
Research Background
Lithium-ion capacitors combine the advantages of secondary batteries and supercapacitors. While having high energy density, they can have both high power density and long cycle life. They are a reliable choice for the next generation of large-scale electrical equipment. However, the inherent imbalance of kinetics between the positive and negative electrodes seriously affects its practical application, and the design and synthesis of a negative electrode material with high rate characteristics is the key to solving this problem. At present, in various types of lithium ion capacitor anode materials, niobium-based oxides stand out by virtue of their fast fast ion insertion-extraction ability, higher theoretical capacity and smaller volume expansion, but common niobium oxides (such as Nb2O5, Ti-Nb-O, etc.) have poor conductivity and unstable crystal structure, which are not conducive to giving full play to the advantages of this type of material. Therefore, it is necessary to explore a niobium oxide negative electrode material with good conductivity and stable structure. Row.
Article Introduction
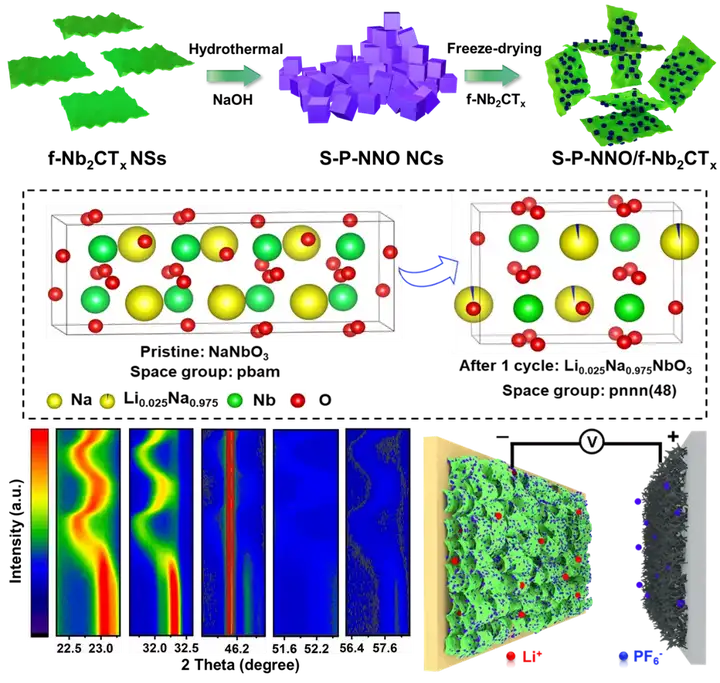
Based on this, Professor Yuan Changzhou and Hou Linrui from the University of Jinan published a titled "Formation and Operating Mechanisms of Single-Crystalline Perovskite NaNbO3 Nanocubes/Few-Layered Nb2CTx MXene Hybrids towards Li-Ion" in the internationally renowned journal Journal of Materials Chemistry A. Capacitors" article. In this article, a small-layer niobium-based MXene (f-Nb2CTx) was used as the precursor to derive a single crystal perovskite sodium niobate nanocube (SP-NNO NCs) by hydrothermal method, and then the niobic acid was constructed by freeze-drying method. Sodium-MXene (SP-NNO/f-Nb2CTx) composite material is used as the negative electrode of lithium ion capacitor. The growth mechanism of S-P-NNO NCs was revealed based on the different hydrothermal reaction time, and the in-situ/ex-situ XRD technology was used to reveal the unique lithium-sodium double-ion intercalation lithium storage mechanism of the composite for the first time.
Highlights of this article
Key point 1: Reveal the growth mechanism of sodium niobate cubes by controlling the different hydrothermal time of f-Nb2CTx MXene nanosheets to reveal the nucleation-growth mechanism of S-P-NNO NCs. In the hydrothermal reaction, the highly active sites on the surface of the f-Nb2CTx MXene nanosheets are easily gradually oxidized to nanoscale Na7(H2O)Nb6O19(H2O)14. As the reaction progressed, the nanosheets were further broken under the attack of NaOH, and more active sites were exposed, forming more and more Na7(H2O)Nb6O19(H2O)14. These formed mesophases use f-Nb2CTx MXene nanosheets as the growth substrate and niobium source, and follow the Oswald maturation growth model to further grow into nanocubes. Finally, all nanosheets are completely converted into S-P-NNO NCs.
Key point 2: The electrochemical characterization of S-P-NNO/f-Nb2CTx composites benefited from the synergistic effect of S-P-NNO NCs and f-Nb2CTx NSs. The composites exhibit ultra-high reversible capacity and excellent rate performance. On the one hand, the existence of MXene nanosheets builds an interconnected conductive network for S-P-NNO NCs, facilitates the insertion of lithium ions, and shortens the diffusion path. On the other hand, the inclusion of S-P-NNO NCs between the MXene sheets can reduce the aggregation and self-accumulation of MXene, which is beneficial to the transport of ions in the electrolyte.
Point 3: Revealing the energy storage mechanism of SP-NNO/f-Nb2CTx composites. In-situ XRD test results show that SP-NNO NCs electrodes have continuous ion insertion/extraction behaviors during charging and discharging. However, it should be noted that in 2 After sub-charge and discharge, part of the diffraction peaks (such as 141 crystal plane) compared with the original state, not only the diffraction angle is slightly shifted, but also the diffraction intensity has not completely recovered to the original state. This is actually because when Li+ was first embedded in NaNbO3 crystals, it exchanged with Na+ in NaNbO3 and transformed into Li0.025Na0.975NbO3 phase. Subsequent ex-situ XRD and EELSs spectra further confirmed this lithium-sodium exchange phenomenon. That is, the lithium storage mechanism of S-P-NNO/f-Nb2CTx is a lithium-sodium double-ion co-intercalation mechanism caused by special ion exchange. This fundamentally explains the real reason why the S-P-NNO NCs electrode cannot be restored to its original state after the lithium ion is released.
Key point four: SP-NNO/f-Nb2CTx//AC lithium ion capacitor characterization SP-NNO/f-Nb2CTx composite as the negative electrode, commercial activated carbon (AC) as the positive electrode, constructed SP-NNO/f-Nb2CTx//AC Lithium ion capacitors. Thanks to the synergistic effect of the intercalation pseudocapacitance characteristics of SP-NNO NCs and the 3D conductive network constructed by MXene, electrons and ions can achieve rapid migration in the electrode, which better overcomes the innate dynamics between the positive and negative electrodes unbalanced. The device can provide an ultra-high energy density of 241 Wh kg1 at 125 W kg1 and a high power density of 13 kW kg1 at an energy density of 55.6 Wh kg1, and a capacity retention rate of 75% after 4000 cycles.
Article link
Formation and Operating Mechanisms of Single-Crystalline Perovskite NaNbO3 Nanocubes/Few-Layered Nb2CTx MXene Hybrids towards Li-Ion Capacitors https://pubs.rsc.org/en/content/articlehtml/2021/ta/d1ta03684j
Corresponding author introduction
Yuan Changzhou, Professor of the School of Materials Science and Engineering, University of Jinan, PhD supervisor, Shandong Province "Taishan Scholar Distinguished Professor", Jinan City Class C Talent (Provincial Leading Talent), Anhui Province Outstanding Youth Fund and Anhui Province Technical Leading Talent By. 2016 ‒ 2020 has been continuously selected into the Clarivate Analytics "Global Highly Cited Scientists" and Aiswell "Chinese Highly Cited Scholars" lists. Won the second prize of the Natural Science Award of the Ministry of Education and one of the Anhui Youth Science and Technology Award. Adhering to the research concept of "materials must be made into materials, materials can be made into devices, and devices are useful", focusing on forward-looking topics and key technical problems in the field of electrochemical storage and conversion, committed to precise synthesis of key materials, structure-component/function control, and internal Basic research on storage/conversion mechanism and device design, construction and optimization. So far, the first/corresponding author has published more than 110 SCI academic papers in international journals such as Angew. Chem. Int. Ed., Adv. Energy Mater., Adv. Funct. Mater., Mater. Today and Mater. Horiz. Articles. Applied for more than 20 Chinese invention patents. He is currently a young editorial board member of Rare Metals and Infomat academic journals.
Introduction of the research group
Group Website: https://www.x-mol.com/groups/Yuan_Changzhou
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.