
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online


The oxidization of MXene under certain conditions is one of the key problems that plague researchers. In this article, today, Professor Yury Gogotsi has made the MXene electrode‘s rate performance greatly controlled by controlled in situ oxidation. Promotion is very informative.
[Background introduction]
MXenes is a rapidly growing two-dimensional nanomaterial that includes transition metal carbides, nitrides and carbonitrides with a combination of hydrophilicity and high electrical conductivity. MXenes usually etch A atoms in a layered MAX phase by selective chemical reaction. M is some early transition metal (Ti, Nb, Mo, V, Cr, Ta, etc.), and A is a 13 or 14 group element (Al, Si). , Ga or In), and X represents a carbon or nitrogen element. MXenes-based composites have variable-valence transition metal oxidation activity, adjustable interlayer spacing, and controlled interface chemical reactions. These advantages allow MXenes-based materials to be widely used in electromagnetic shielding and energy storage. MXenes materials store charge in the form of intercalated tantalum capacitors. The oxidation reaction is accompanied by ultra-fast ion reaction kinetics, which is quite different from the slow ion diffusion of ordinary battery materials and the electric double layer formed on the surface of porous carbon electrodes. The ability of multiple ions to be rapidly embedded in the MXenes layer makes it possible to prepare high-magnification electrode materials for hybrid metal ion capacitors and multivalent batteries.
[Introduction]
Recently, Professor Yury Gogotsi of Drexel University in the United States reported a controlled in situ anodized Ti3C2Tx electrode with good electrochemical properties. This controlled anodization improves the rate performance of the Ti3C2Tx MXene electrode in an acidic electrolyte environment. Depending on the degree of controllable oxidation, the capacity retention rate increased from 38% to 66% as the scan rate increased from 5 mV s-1 to 2000 mV s-1. At the same time, the higher anode potential after oxidation demonstrates that the redox reaction is somewhat attenuated. The chemical changes of this structure and interface were proved by some column analysis methods. Introducing defects is a key factor to improve the performance of Ti3C2Tx rate without losing the electrochemical active sites.
The results were published online Angew. Chem. Int. Ed: Tuning the Electrochemical Performance of Titanium Carbide MXene by Controllable In Situ Anodic Oxidation.
[Graphic introduction]
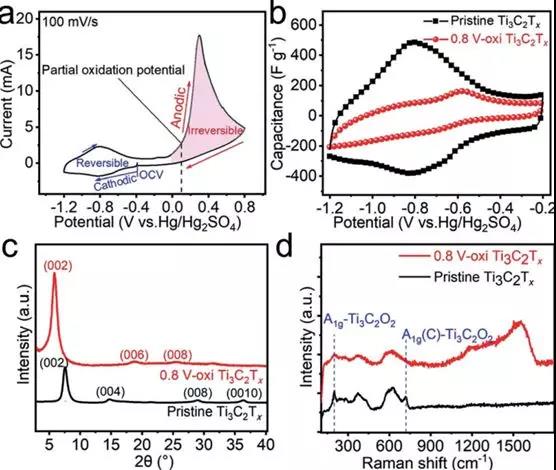
Figure 1 a) CV image of a reversible and irreversible electrochemical process with a sweep rate of 100 mV/s Ti3C2Tx at a stable voltage window b) Contrast image of pure Ti3C2Tx with CV at 0.8V oxidation potential. c) Ex situ XRD and d) Raman maps.

Figure 2 Electrochemical performance of Ti3C2Tx electrode under different oxidation conditions.

Figure 3 Exploring the excellent rate performance mechanism of partially oxidized Ti3C2Tx MXene electrode: a) XRD image at different potentials; b) - c) TEM image, d) in situ Raman spectrum, e) XPS; f) Schematic diagram of oxidation process.

Figure 4 Oxidation Ti3C2Tx Ragone-plots image. a) after 0.1V multiple oxidation cycle; b) after one oxidation process.
[Summary of this article]
The enhanced electrochemical performance of Ti3C2TxMXene is achieved by a controlled electrochemical oxidation process. Multiple cycles of the electrode cause the anode potential to be slightly higher than the conventional operating voltage, widening the layer spacing of the two-dimensional nanosheets and introducing a porous structure in the MXene layer without destroying the electrochemically active sites. Compared to the pure MXene electrode, the optimal partial oxidation control doubles the capacity retention of Ti3C2Tx in acidic electrolytes at very high sweep rates because the kinetics of the oxidized Ti3C2Tx electrode is increased by about 30%.
Literature link:
https://doi.org/10.1002/anie.201911604
DOI: 10.1002/anie.201911604
Source: WeChat public number MXene Frontier

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |